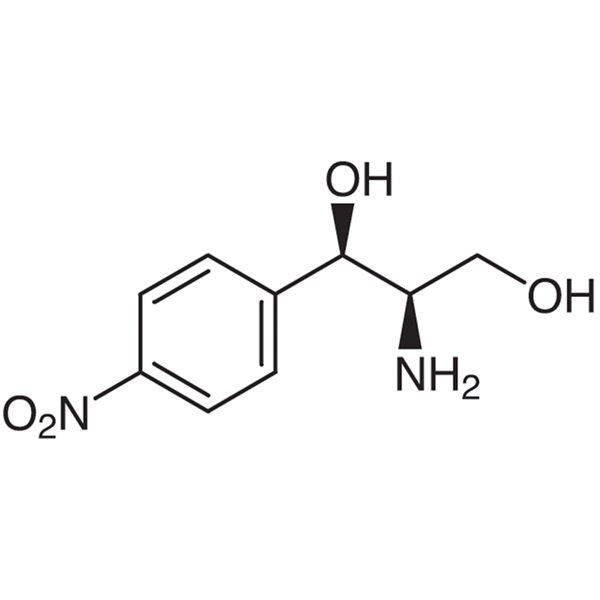
(1R,2R)-(-)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol CAS 716-61-0 Purity ≥99.0% (HPLC) High Purity
Manufacturer Supply with High Purity and Commercial Production
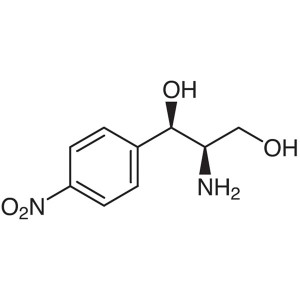
(1R,2R)-(-)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol CAS 716-61-0
(1S,2S)-(+)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol CAS 2964-48-9
Chloramphenicol CAS 56-75-7
| Chemical Name | (1R,2R)-(-)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol |
| Synonyms | D-(-)-threo-2-Amino-1-(4-nitrophenyl)-1,3-propanediol; Chloramphenico L-Base; L-Base; Levoamine |
| CAS Number | 716-61-0 |
| CAT Number | RF-CC292 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C9H12N2O4 |
| Molecular Weight | 212.2 |
| Solubility | Soluble in Water, Ethanol, Methanol, DMF, DMSO |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Pale Yellow Powder |
| Melting Point | 160.0~165.0℃ |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.50% |
| Optical Rotation [a]20D | -28.5° ~ -30.5° |
| Moisture (K.F) | ≤0.50% |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates; Chloramphenico L-Base |
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (1R,2R)-(-)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol (CAS: 57794-08-8), also named D-(-)-threo-2-Amino-1-(4-nitrophenyl)-1,3-propanediol with high quality.
(1R,2R)-(-)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol (CAS: 57794-08-8) (Chloramphenicol base) is an intermediate for synthesizing Chloramphenicol, a broad spectrum antibiotic agent. Chloramphenicol base is the parent 4-nitrophenylpropylamine formed by the hydrolysis of the dichloroacetamide of chloramphenicol. Chloramphenicol base per se does not work as an antibiotic, however, it plays an important role in the synthesis and antibacterial activities of chloramphenicol and other new generation antibiotics, represented by thiamphenicol and its experimental analogues, bromamphenicol and methamphenicol.