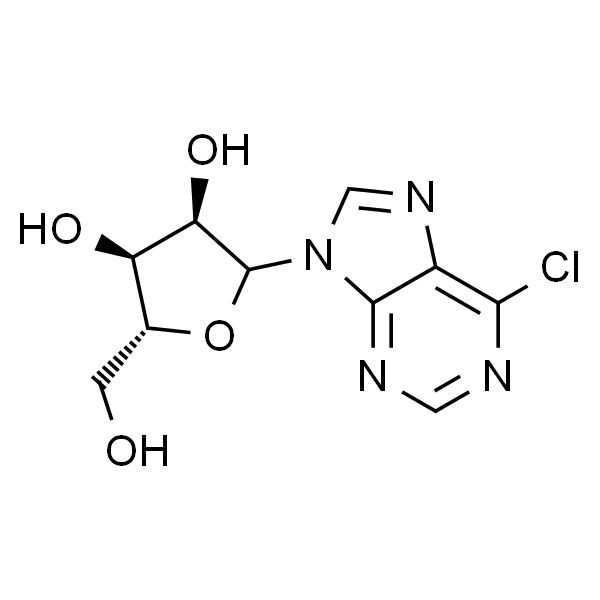
6-Chloropurine Ribonucleoside CAS 2004-06-0 Purity ≥99.0% (HPLC) Factory
Manufacturer Supply, High Purity, Commercial Production
Chemical Name: 6-Chloropurine Ribonucleoside
CAS: 2004-06-0
| Chemical Name | 6-Chloropurine Ribonucleoside |
| Synonyms | 6-CLPR; 6-Chloropurine Riboside; 6-Chloropurine-9-Riboside |
| CAS Number | 2004-06-0 |
| CAT Number | RF-PI506 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H11ClN4O4 |
| Molecular Weight | 286.67 |
| Melting Point | 158.0 to 162.0℃ (dec.)(lit.) |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Light Yellow Crystalline Powder |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤10ppm |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Storage | Store in cool and dry place, 2-8℃ |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Items of Analysis
Description: Light Yellow Crystalline Powder (View by Eye)
Loss on Drying: NMT 0.50% w/w (100℃ 4h)
Residue on Ignition: NMT 0.10% (According to Residue on Ignition, CP2005 Excursus 8 N) .
Heavy Metals: NMT 10ppm (According to Method Ⅱ of Heavy Metals, CP2005 Excursus 8 H) .
Chromatographic Purity (HPLC): NLT 99.0%
Instrument: SPD-10Avp Column: VP-ODS 250L*4.6
Mobile Phase: CH3CN:H2O=1:9
Flow Rate: 1.0mL/min Wavelength: 254nm
Procedure: approximately 0.02 g to 100-mL volumetric flask, dissolve and dilute with mobile phase to volume, and mix as the test solution. Then inject about 20μL of the test solution. Determine the percentage of the major peak by area normalization,record the chromatograms and calculate the peak responses for the major peaks.
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


6-Chloropurine Ribonucleoside (CAS: 2004-06-0) is used an intermediate in organic synthesis, synthesis of pharmaceutical intermediates and Active Pharmaceutical Ingredient (API).
-
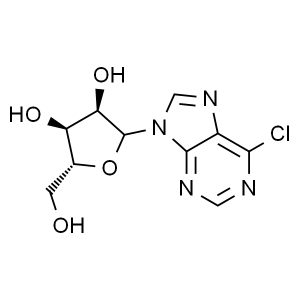
6-Chloropurine Ribonucleoside CAS 2004-06-0 Pur...
-
6-Chloropurine 6-CP CAS 87-42-3 Assay ≥99.0% (H...
-
6-Chloropurine Riboside CAS 5399-87-1 Assay ≥98...
-
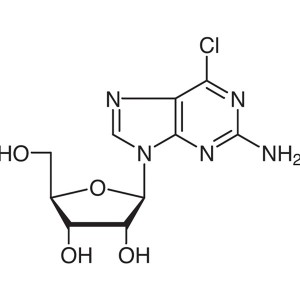
2-Amino-6-Chloropurine Riboside CAS 2004-07-1 A...
-
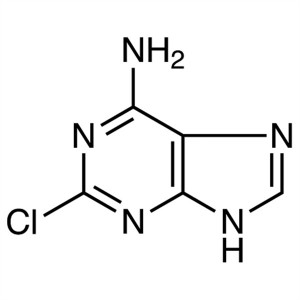
2-Chloroadenine CAS 1839-18-5 Assay ≥98.0% (HPL...
-
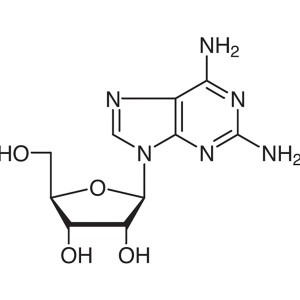
2-Aminoadenosine CAS 2096-10-8 Purity ≥99.0% (H...