7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one CAS 160129-45-3 Tolvaptan Intermediate
Manufacturer Supply, High Quality, Commercial Production
Tolvaptan and Related Intermediates:
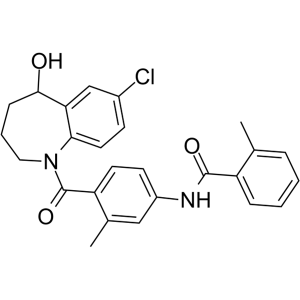
Tolvaptan CAS 150683-30-0
7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one CAS 160129-45-3
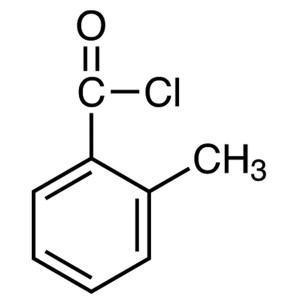
o-Toluoyl Chloride CAS 933-88-0
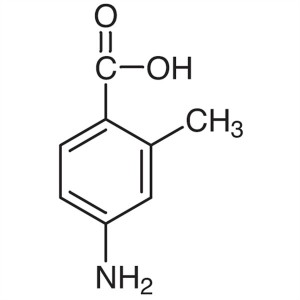
4-Amino-2-Methylbenzoic Acid CAS 2486-75-1
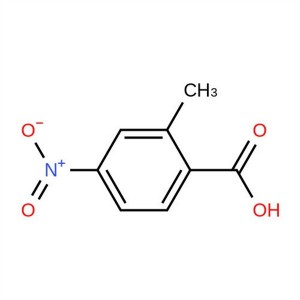
2-Methyl-4-Nitrobenzoic Acid CAS 1975-51-5
| Chemical Name | 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one |
| Synonyms | 7-Chloro-1,2,3,4-tetrahydro-5H-1-benzazepin-5-one; 7-Chloro-3,4-dihydro-1H-benzo[b]azepin-5(2H)-one |
| CAS Number | 160129-45-3 |
| CAT Number | RF-PI395 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H10ClNO |
| Molecular Weight | 195.65 |
| Melting Point | 103.0 to 107.0℃ |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Light Green to Light Yellow Powder |
| Identification Methods | NMR, HPLC |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.50% |
| Any Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Tolvaptan (CAS 150683-30-0), treatment of Hyponatremia |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one (CAS 160129-45-3) is an intermediate in the synthesis of Tolvaptan (CAS 150683-30-0). Tolvaptan is a selective, competitive orally active nonpeptide arginine vasopressin V2 receptor antagonist with an IC50 of 1.28µM for the inhibition of AVP-induced platelet aggregation. Tolvaptan is used to treat hyponatremia associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone. Tolvaptan is also in fast-track clinical trials for polycystic kidney disease. Treatment with Tolvaptan causes rapid and sustained body weight reductions concurrent with increases in urine output, improves and/or normalizes serum sodium in hyponatremic patients, reduces signs and symptoms of congestion and increases thirst.

![7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one CAS 160129-45-3 Tolvaptan Intermediate Featured Image](https://www.ruifuchem.com/uploads/7-Chloro-1234-tetrahydrobenzobazepin-5-one-CAS-160129-45-3.jpg)
![7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one CAS 160129-45-3 Tolvaptan Intermediate](https://www.ruifuchem.com/uploads/7-Chloro-1234-tetrahydrobenzobazepin-5-one-CAS-160129-45-3-300x300.jpg)