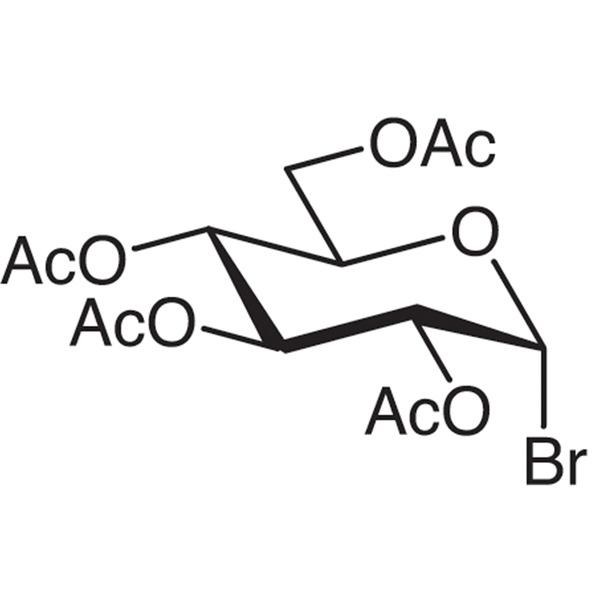
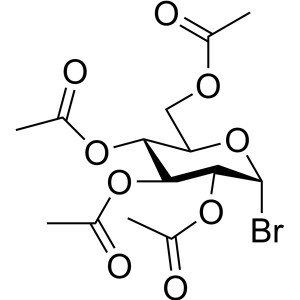
Acetobromo-α-D-Glucose CAS 572-09-8 Purity >99.0% (HPLC) Factory
Ruifu Chemical is the leading manufacturer of 2,3,4,6-Tetra-O-Acetyl-α-D-Glucopyranosyl Bromide (Acetobromo-α-D-Glucose) (CAS: 572-09-8) with high quality, stabilized with CaCO3. Ruifu Chemical has 15 years experience in carbohydrate chemistry. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | 2,3,4,6-Tetra-O-Acetyl-α-D-Glucopyranosyl Bromide |
| Synonyms | 2,3,4,6-Tetra-O-Acetyl-alpha-D-Glucopyranosyl Bromide; Acetobromo-α-D-Glucose; Acetobromo-alpha-D-Glucose; α-Acetobromoglucose; 1-Bromo-α-D-Glucose Tetraacetate; TAGB |
| Stabilizer | Stabilized with 2% CaCO3 |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 572-09-8 |
| Molecular Formula | C14H19BrO9 |
| Molecular Weight | 411.20 g/mol |
| Melting Point | 87.0 to 92.0℃ |
| Density | 1.49±0.10 g/cm3 |
| Store Under Inert Gas | Store Under Inert Gas (e.g. Argon) |
| Sensitive | Light Sensitive, Moisture Sensitive, Heat Sensitive |
| Water Solubility | Decomposes |
| Storage Temp. | Store at ≤ -10℃; Shipped in Dry Ice |
| Stability | Stable. Combustible. Incompatible with Strong Oxidizing Agents |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | Carbohydrate Derivative |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Crystalline Powder | White Crystalline Powder |
| Purity / Analysis Method | >99.0% (by HPLC) | 99.35% |
| Assay / Analysis Method | >99.0% (by Titration) | 99.41% |
| Melting Point | 87.0~92.0℃ | 89.3℃ |
| Water by Karl Fischer | <0.50% | <0.50% |
| Specific Rotation [a]20/D | +197.0° ± 5.0° (C=1 in CHCl3) | +198.5° |
| TLC | One Spot | One Spot |
| 1H NMR | Conforms to Structure | Complies |
| Stabilizer | 2% CaCO3 | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (-10℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture. Store away from oxidizing agents, acids.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description S24/25 - Avoid contact with skin and eyes.
S36 - Wear suitable protective clothing.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany -
FLUKA BRAND F CODES 8-10-21
HS Code 2940009090
2,3,4,6-Tetra-O-Acetyl-α-D-Glucopyranosyl Bromide (Acetobromo-α-D-Glucose) (CAS: 572-09-8) is a carbohydrate derivative and a chemical reagent extensively used in glycochemistry as a glycosyl donor or acceptor in the synthesis of various oligosaccharide compounds. The compound is also used in the synthesis of glycopeptides, glycoproteins, and carbohydrates with potential biological activity. It is used to prepare glycosylated natural products and provides access to complex carbohydrate structures for biological studies. It acts as an intermediate in the preparation of beta-glucosides. It is commercially available and widely used in glycochemistry research.
2,3,4,6-Tetra-O-Acetyl-α-D-Glucopyranosyl Bromide has also been used in the synthesis of oligosaccharide compounds that have biological activity.
2,3,4,6-Tetra-O-Acetyl-α-D-Glucopyranosyl Bromide is used as a reagent in organic synthesis, as a building block in the synthesis of carbohydrates and other molecules, and as a substrate in enzymatic reactions. It is also used as a model compound in the study of enzyme kinetics and substrate specificity.
There are several potential future directions for research involving 2,3,4,6-Tetra-O-Acetyl-α-D-Glucopyranosyl Bromide. It could be used to study the interactions between carbohydrates and proteins, as well as to synthesize novel carbohydrates and other molecules. It could also be used to study the mechanisms of glycosyltransferases and glycosidases. Additionally, it could be used in the study of enzyme kinetics and substrate specificity. Finally, it could be used to develop new therapeutic agents for the treatment of various diseases.
2,3,4,6-Tetra-O-Acetyl-α-D-Glucopyranosyl Bromide (Acetobromo-α-D-Glucose) (CAS: 572-09-8), Despite the broad applicability of this compound in glycochemistry research, it has limitations, including lower reaction yields, increased reaction times, and low stereoselectivity. Future research should focus on overcoming these challenges and streamlining the synthesis process while maintaining high efficiency. Other future directions include the usage of this compound in drug design and its application in synthetic glycobiology. Newer methodologies to generate and transform glycosides should also be developed, keeping the safety concerns in mind.
-
Acetobromo-α-D-Glucose CAS 572-09-8 Purity >99....
-
2,3,4,6-Tetra-O-Benzyl-D-Glucopyranose CAS 4132...
-
2,3,4,6-Tetra-O-Benzyl-α-D-Glucopyranose CAS 65...
-
1,2-O-Isopropylidene-α-D-Glucofuranose CAS 1854...
-
1,2-O-Isopropylidene-α-D-Xylofuranose CAS 20031...
-
1,3,5-Tri-O-benzoyl-D-Ribofuranose CAS 22224-41...
-
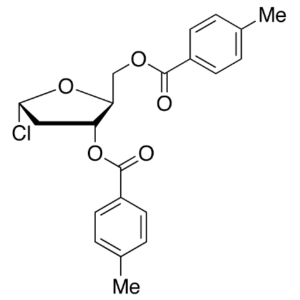
1-Chloro-2-Deoxy-3,5-Di-O-Toluoyl-L-Ribofuranos...
-
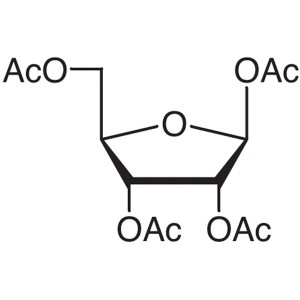
β-D-Ribofuranose 1,2,3,5-Tetraacetate CAS 13035...
-
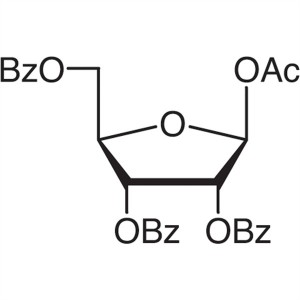
β-D-Ribofuranose 1-Acetate 2,3,5-Tribenzoate CA...
-
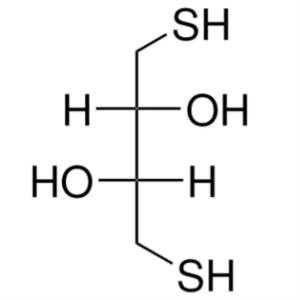
DL-Dithiothreitol (DTT) CAS 3483-12-3 Assay >98...
-
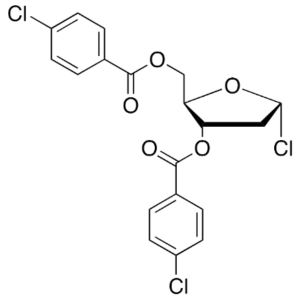
1-Chloro-3,5-Di-(4-Chlorobenzoyl)-2-Deoxy-D-Rib...
-
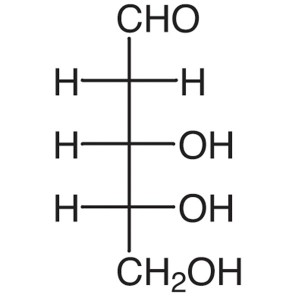
2-Deoxy-D-Ribose CAS 533-67-5 Assay >98.0% (HPL...
-
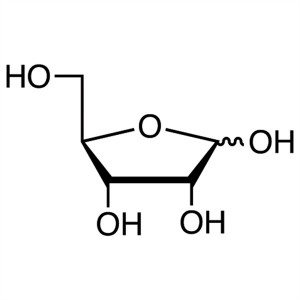
D-(-)-Ribose CAS 50-69-1 Assay 97.0~102.0% Fact...
-
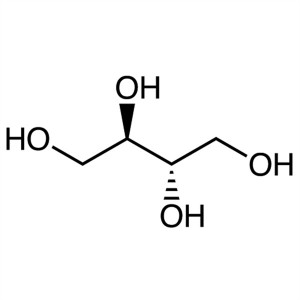
meso-Erythritol CAS 149-32-6 Assay 99.5~100.5% ...
-
D-(+)-Galactosamine Hydrochloride CAS 1772-03-8...
-
N-Octyl-D-Glucamine CAS 23323-37-7 Purity >98.0...