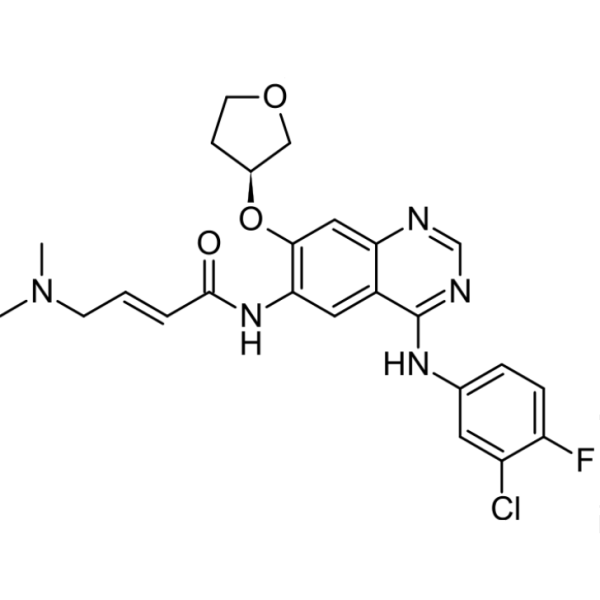
Afatinib CAS 439081-18-2 Purity >99.5% (HPLC) Factory
Ruifu Chemical Supply Intermediates of Afatinib
Afatinib CAS 439081-18-2
Afatinib Dimaleate CAS 850140-73-7
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2
(Dimethylamino)acetaldehyde Diethyl Acetal CAS 3616-56-6
trans-4-Dimethylaminocrotonic Acid Hydrochloride CAS 848133-35-7
Diethylphosphonoacetic Acid CAS 3095-95-2
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012-69-3
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449-14-2
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine CAS 162012-67-1
(S)-N4-(3-Chloro-4-Fluorophenyl)-7-((Tetrahydrofuran-3-yl)oxy)quinazoline-4,6-Diamine CAS 314771-76-1
(S)-N-(3-Chloro-4-Fluorophenyl)-6-Nitro-7-((Tetrahydrofuran-3-yl)oxy)quinazolin-4-Amine CAS 314771-88-5
| Chemical Name | Afatinib |
| Synonyms | BIBW2992; BIBW-2992; BIBW2992 Free Base; Tomtovok; (S,E)-N-(4-(3-Chloro-4-Fluorophenylamino)-7-(Tetrahydrofuran-3-yloxy)quinazolin-6-yl)-4-(Dimethylamino)but-2-Enamide |
| CAS Number | 439081-18-2 |
| CAT Number | RF-PI2033 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C24H25ClFN5O3 |
| Molecular Weight | 485.94 |
| Solubility | Soluble in DMSO |
| Density | 1.380 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Off-White Powder |
| Identification | HPLC, NMR |
| Purity / Analysis Method | >99.5% (HPLC) |
| Melting Point | 100.0~102.0℃ |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Total Impurities | <0.50% |
| Heavy Metals | ≤20ppm |
| NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Shelf Life | 24 Months if Stored Properly |
| Usage | API; Afatinib; Afatinib Dimaleate; NSCLC |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Afatinib, also known as BIW-2992, (CAS: 439081-18-2) is the second-generation potent and irreversible dual inhibitor of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) tyrosine kinase, developed by Boehringer Ingelheim, Germany. It is capable of irreversibly inhibiting the activity of the tyrosine kinase by undergoing the Michael reaction with the thiol group of cysteine at position 797 of the EGFR. On July 12, 2013, it became a new drug for anti-small cell lung cancer approved by the US FDA under the trade name of Gilotrif. This drug is a tablet. It is used for the treatment of the patients diagnosed with metastatic non-small cell lung cancer (NSCLC) with the loss of the 19th exon or L858R mutation in the 21th exon of the tumor epidermal growth factor receptor (EGFR) confirmed using the kit approved by FDA. The drug is also effective in the treatment of HER2-positive patients with advanced breast cancer. Afatinib belongs to a class of drugs known as tyrosine kinase inhibitors. Tyrosine kinase inhibitors are designed to block the action of a specific enzyme called tyrosine kinase. This enzyme plays a big role in the function of cells, and is active in promoting tumor growth and progression. Afatinib works to inhibit the function of two types of tyrosine kinases: epidermal growth factor receptor (EGFR) and Her2, which are "over-expressed" by several types of cancer. By blocking the function of these tyrosine kinases, Afatinib may prevent cancer cells from dividing and growing.
-
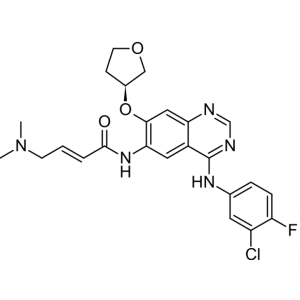
Afatinib CAS 439081-18-2 Purity >99.5% (HPLC) F...
-
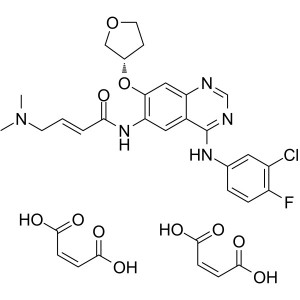
Afatinib Dimaleate CAS 850140-73-7 Purity >99.5...
-
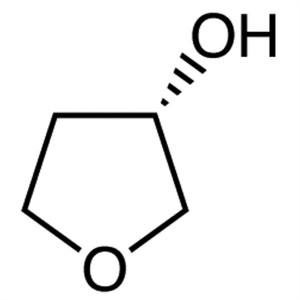
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2...
-
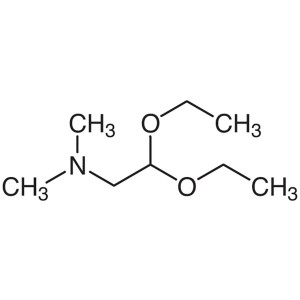
(Dimethylamino)acetaldehyde Diethyl Acetal CAS ...
-
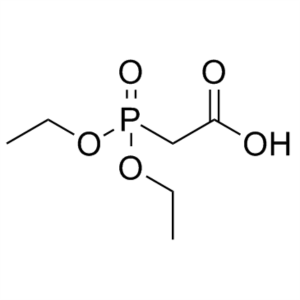
Diethylphosphonoacetic Acid CAS 3095-95-2 Purit...
-
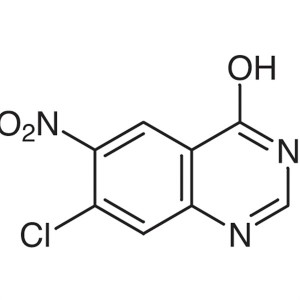
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449...
-
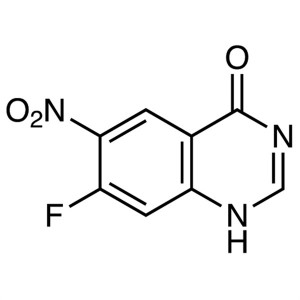
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012...
-
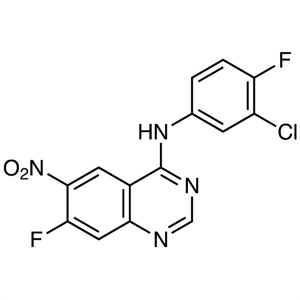
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroqui...
-
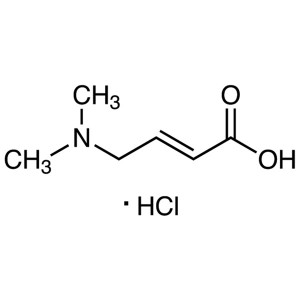
trans-4-Dimethylaminocrotonic Acid Hydrochlorid...
-
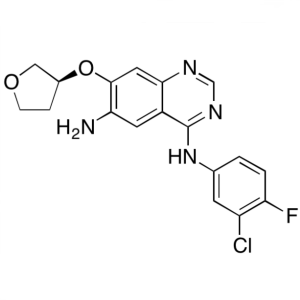
Afatinib Dimaleate Intermediate CAS 314771-76-1...
-
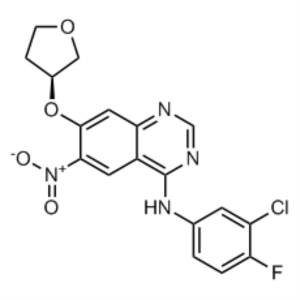
Afatinib Dimaleate Intermediate CAS 314771-88-5...