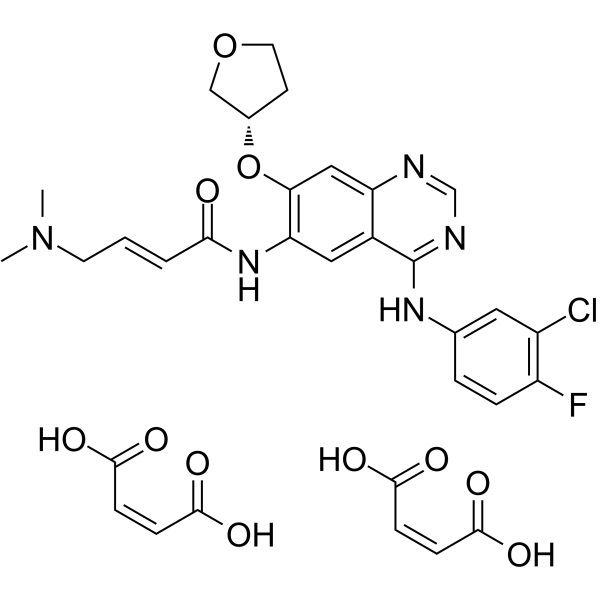
Afatinib Dimaleate CAS 850140-73-7 Purity >99.5% (HPLC) API
Ruifu Chemical Supply Intermediates of Afatinib
Afatinib CAS 439081-18-2
Afatinib Dimaleate CAS 850140-73-7
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2
(Dimethylamino)acetaldehyde Diethyl Acetal CAS 3616-56-6
trans-4-Dimethylaminocrotonic Acid Hydrochloride CAS 848133-35-7
Diethylphosphonoacetic Acid CAS 3095-95-2
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012-69-3
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449-14-2
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine CAS 162012-67-1
(S)-N4-(3-Chloro-4-Fluorophenyl)-7-((Tetrahydrofuran-3-yl)oxy)quinazoline-4,6-Diamine CAS 314771-76-1
(S)-N-(3-Chloro-4-Fluorophenyl)-6-Nitro-7-((Tetrahydrofuran-3-yl)oxy)quinazolin-4-Amine CAS 314771-88-5
| Chemical Name | Afatinib Dimaleate |
| Synonyms | BIBW2992 Dimaleate; (S,E)-N-(4-(3-Chloro-4-Fluorophenylamino)-7-(Tetrahydrofuran-3-yloxy)quinazolin-6-yl)-4-(dimethylamino)but-2-Enamide Dimaleate |
| CAS Number | 618-89-3 |
| CAT Number | RF-PI2032 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C32H33ClFN5O11 |
| Molecular Weight | 718.08 |
| Sensitivity | Moisture Sensitive |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Purity / Analysis Method | >99.5% (HPLC) |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.10% |
| Maximum Single Impurity | <0.30% |
| Total Impurities | <0.50% |
| Heavy Metals (as Pb) | ≤20ppm |
| Infrared Spectrum | Conforms to Structure |
| NMR | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Shelf Life | 24 Months if Stored Properly |
| Usage | API |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Afatinib Dimaleate (CAS: 850140-73-7), the dimaleate salt form of afanitib, is an orally available antineoplastic agent. Afatinib Dimaleate is an irreversible EGFR family inhibitor with IC50s of 0.5 nM, 0.4 nM, 10 nM and 14 nM for EGFRwt, EGFRL858R, EGFRL858R/T790M and HER2, respectively. Afatinib Dimaleate is indicated for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. In the past, standard treatment with a platinum-based chemotherapy doublet regimen was considered standard first-line therapy for all patients with NSCLC. However, emerging evidence has identified subpopulations in which targeted therapy is more effective, leading to the development of mutation-specific drugs. Afatinib Dimaleate was Developed by Boehringer Ingelheim Pharmaceuticals, Afatinib Dimaleate was approved by the FDA in 2013 as an orphan drug under the trade name Gilotrif. Afatinib Dimaleate is chemically synthesized using standard methods. Afatinib Dimaleate is not only active against EGFR mutations targeted by first generation TKIs like erlotinib or gefitinib, but also against mutations such as T790M which are not sensitive to these standard therapies. Because of its additional activity against Her2, it is being investigated for breast cancer as well as other EGFR and Her2 driven cancers.
-
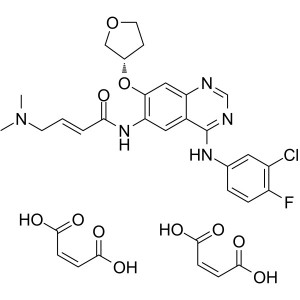
Afatinib Dimaleate CAS 850140-73-7 Purity >99.5...
-
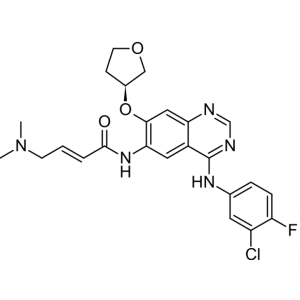
Afatinib CAS 439081-18-2 Purity >99.5% (HPLC) F...
-
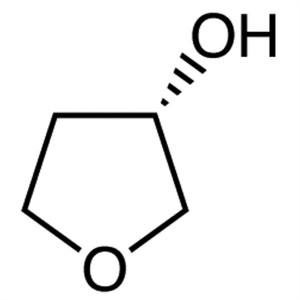
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2...
-
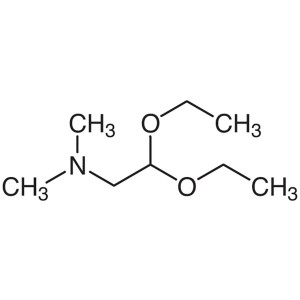
(Dimethylamino)acetaldehyde Diethyl Acetal CAS ...
-
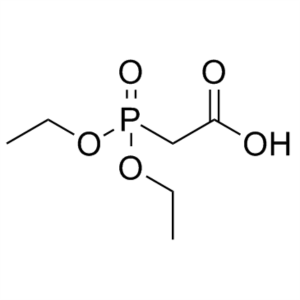
Diethylphosphonoacetic Acid CAS 3095-95-2 Purit...
-
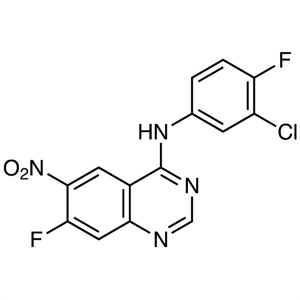
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroqui...
-
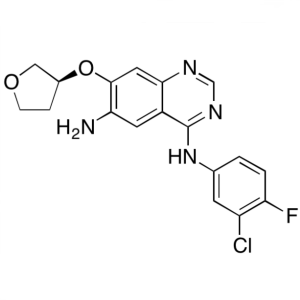
Afatinib Dimaleate Intermediate CAS 314771-76-1...
-
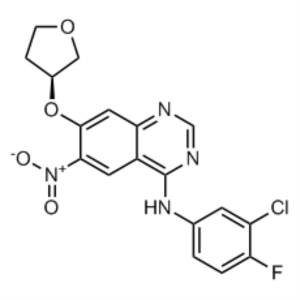
Afatinib Dimaleate Intermediate CAS 314771-88-5...
-
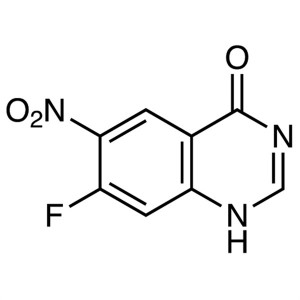
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012...
-
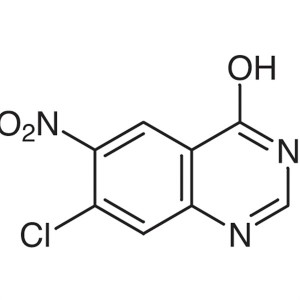
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449...
-
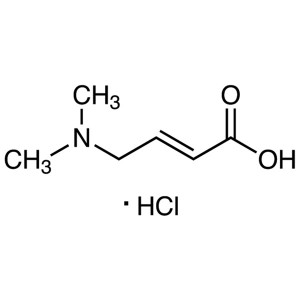
trans-4-Dimethylaminocrotonic Acid Hydrochlorid...