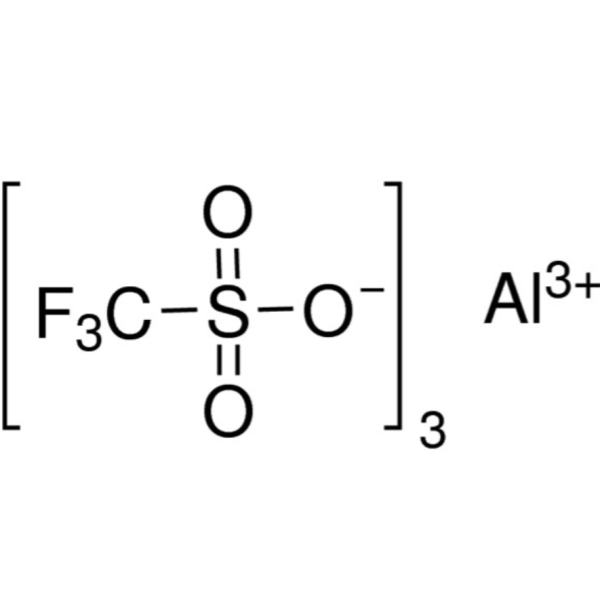
Aluminum Trifluoromethanesulfonate CAS 74974-61-1 Purity >98.0% Aluminum 5.3~6.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Aluminum Trifluoromethanesulfonate (CAS: 74974-61-1) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | Aluminum Trifluoromethanesulfonate |
| Synonyms | Aluminum Triflate; Trifluoromethanesulfonic Acid Aluminum Salt ; Al(OTf)3 |
| CAS Number | 74974-61-1 |
| CAT Number | RF-PI2110 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | (CF3SO3)3Al |
| Molecular Weight | 474.19 |
| Sensitivity | Moisture Sensitive, Hygroscopic |
| Melting Point | 300℃ (lit.) |
| Water Solubility | Insoluble in Water |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White Powder |
| Purity | >98.0% |
| Aluminum | 5.3~6.0% (Complexometric EDTA) |
| Infrared Spectrum | Conforms to Structure |
| ICP Major Analysis | Confirms Al & S Components Confirmed |
| Test Standard | Enterprise Standard |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Aluminum Trifluoromethanesulfonate (CAS: 74974-61-1), Lewis acid used as a catalyst for Friedel-Crafts, ketalization, nucleophilic substitution, hydroalkoxylation, methoxycarbonyla- tion, rearrangement, and epoxide ring-opening reactions. Aluminum Trifluoromethanesulfonate is used in pharmaceutical intermediates. Aluminum Trifluoromethanesulfonate can be employed as a catalyst: In the regioselective synthesis of cyclic ethers by cycloisomerization of unsaturated alcohols In the conversion of saccharides into 5-hydroxymethylfurfural (5-HMF). Along with Pd(OAc)2/BINAP for the methoxycarbonylation reaction of phenylacetylene. Aluminum Trifluoromethanesulfonate has been used for the Friedel-Crafts alkylation reaction of toluene with isopropyl and tert-butyl chlorides (eq 1), and for theacylationofbenzeneandtoluenewithacetylandbenzoylchlo- rides in low to moderate yields.
-
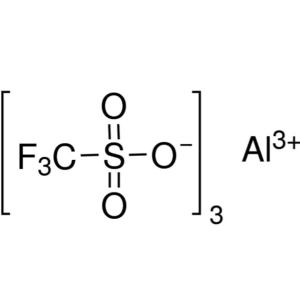
Aluminum Trifluoromethanesulfonate CAS 74974-61...
-
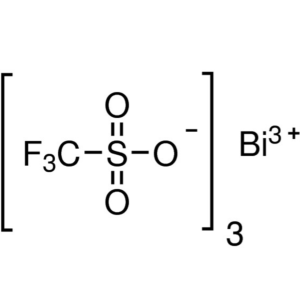
Bismuth(III) Trifluoromethanesulfonate CAS 8818...
-
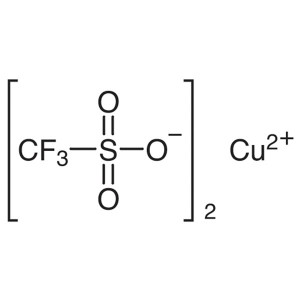
Copper(II) Trifluoromethanesulfonate CAS 34946-...
-
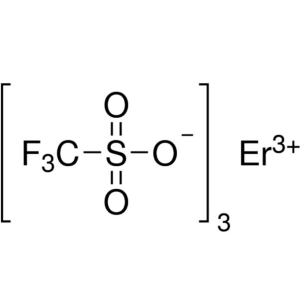
Erbium(III) Trifluoromethanesulfonate CAS 13917...
-
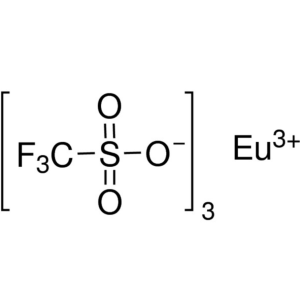
Europium(III) Trifluoromethanesulfonate CAS 520...
-
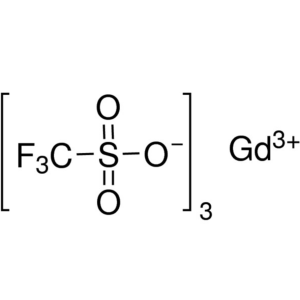
Gadolinium(III) Trifluoromethanesulfonate CAS 5...
-
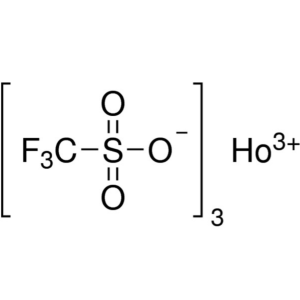
Holmium(III) Trifluoromethanesulfonate CAS 1391...
-
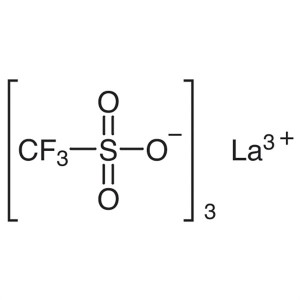
Lanthanum(III) Trifluoromethanesulfonate CAS 52...
-
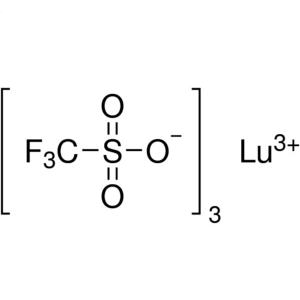
Lutetium(III) Trifluoromethanesulfonate CAS 126...
-
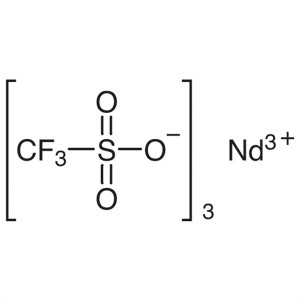
Neodymium(III) Trifluoromethanesulfonate CAS 34...
-
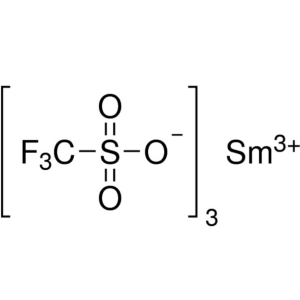
Samarium(III) Trifluoromethanesulfonate CAS 520...
-
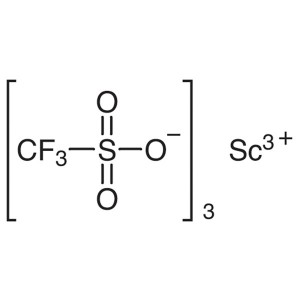
Scandium(III) Trifluoromethanesulfonate CAS 144...
-
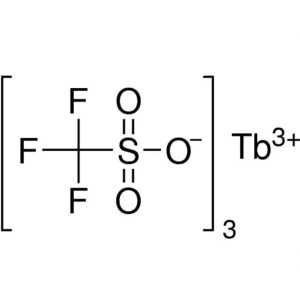
Terbium(III) Trifluoromethanesulfonate CAS 1489...
-
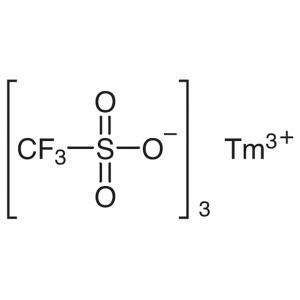
Thulium(III) Trifluoromethanesulfonate CAS 1414...
-
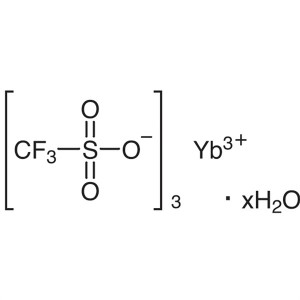
Ytterbium(III) Trifluoromethanesulfonate Hydrat...
-
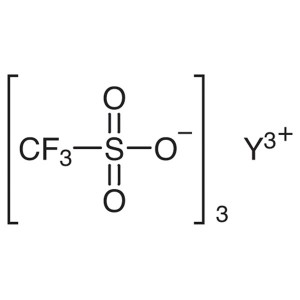
Yttrium(III) Trifluoromethanesulfonate CAS 5209...