Best Price for Fingolimod HCl - Rosuvastatin Calcium CAS 147098-20-2 High Purity – Ruifu
Best Price for Fingolimod HCl - Rosuvastatin Calcium CAS 147098-20-2 High Purity – Ruifu Detail:
High Purity and Competitive Price
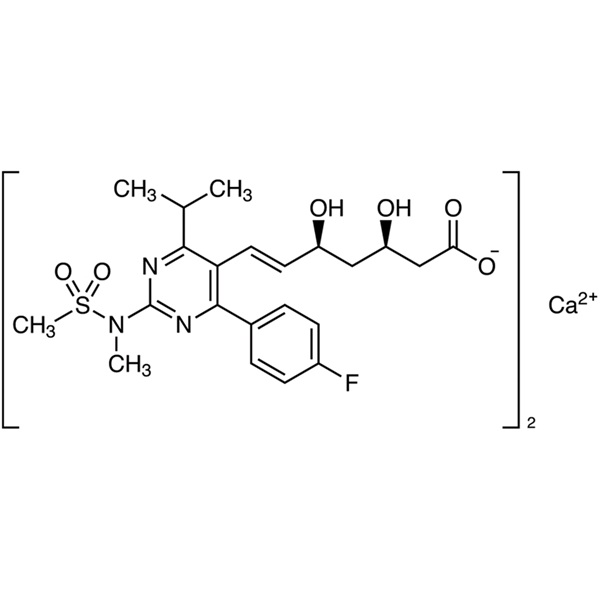
Rosuvastatin Calcium
CAS: 147098-20-2
Appearance: White to Yellow Powder
Calcium Content: 3.5%~4.5%
Purity: ≥98.0%
Moisture (K.F): ≤5.0%
| Chemical Name | Rosuvastatin Calcium |
| CAS Number | 147098-20-2 |
| CAT Number | RF-API01 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C44H54CaF2N6O12S2 |
| Molecular Weight | 1001.14 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Light Yellow Powder |
| Calcium Content | 3.5%~4.5% |
| Moisture (K.F) | ≤5.0% |
| Heavy Metal | ≤0.002% |
| Test Standard | Enterprise Standard |
| Usage | API |
Package: Bottle, Barrel, Cardboard drum, 25kg/Barrel, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Anti-hyperlipidemia drugs
Rosuvastatin Calcium (CAS: 147098-20-2) is an anti-hyperlipidemia drug which belongs to the inhibitor of HMG-CoA reductase successfully developed by the British AstraZeneca company. It is suitable for the treatment of various lipid abnormalities, including hypercholesterolemia, mixed lipid qualitative abnormalities and simple hypertriglyceridemia. Rosuvastatin Calcium (CAS: 147098-20-2) is listed as the strongest and the most comprehensive statins drugs which has entered into market for lipid-lowering and adjustment. Compared with atorvastatin which is currently recognized with the world’s best efficacy, it has a better effect of lowering the level of LDL cholesterol and increasing the level of high-density lipoprotein. Moreover, it has a better tolerance, lower side effects and unique pharmacokinetic characteristics with the half-life about 20h. It only needs to be taken once a day.
Pharmacological effects
Rosuvastatin Calcium (CAS: 147098-20-2) is a selective inhibitor of HMG-CoA reductase. HMG-CoA reductase inhibitor is the rate-limiting enzyme of the transition hydroxy-3-methylglutaryl coenzyme A to valerate A (The precursor of cholesterol). The main site of action of rosuvastatin is liver-the target organs of cholesterol-lowering. Rosuvastatin increases the number of hepatic LDL receptors on the cell surface, promoting the absorption and catabolism of LDL, inhibits hepatic synthesis of VLDL, thereby reducing the total number of VLDL and LDL particles.
For patients of homozygous and heterozygous familial hypercholesterolemia, non-familial hypercholesterolemia, and mixed dyslipidemia, Rosuvastatin can lower the total cholesterol, LDL-C levels, ApoB levels, non-HDL-C levels. Rosuvastatin also reduces the level of TG and increase the level of HDL-C. For patients of pure hypertriglyceridemia, rosuvastatin can lower total cholesterol, LDL-C, VLDL-C, ApoB, non-HDL-C, TG levels and increase the HDL-C levels.
Based on the general safety of pharmacology, repeated dose toxicity, potential genetic toxicity and the clinical information of carcinogenicity, no special toxicity was found for rosuvastatin on the human body. For studies on rat before and after birth, Rosuvastatin has obvious reproductive toxicity. It reduces the size, weight of the litter and birth rate. Treating female rats with a toxic dose which causes several times higher systemic exposure levels than the therapeutic exposure can enable the observation of these phenomena.
The drug can be absorbed in great amount by liver after oral administration with the volume of distribution at 134 L. After 3~5 hours, plasma concentration reaches the peak. The absolute bioavailability is around 20%. Plasma protein binding rate (mainly albumin) is about 90%. About 90% of the total dose of rosuvastatin is excreted by feces as prototype (including the absorption of the active substance and unabsorbed), with the rest excreted through the urine. 5% rosuvastatin in urine is in the form of prototype. Half-life of plasma elimination is about 19 hours. Elimination half-life doesn’t increase with increased dose. The geometric mean of plasma clearance rate is about 50L/hour (coefficient of variation 21.7%). Same as other HMG-CoA reductase inhibitors, the uptake of rosuvastatin by liver involves the participation of membrane transporter OATP-C. The transporter is important in the liver for the clearance of rosuvastatin.
Drug Interactions
1. Cyclosporine: when this product is used in combination with cyclosporine, the AUC of rosuvastatin is averagely 7 times higher than that observed in healthy volunteers (compared with the same dose using this service). Combination usage does not affect the plasma concentrations of cyclosporine.
2. Vitamin K antagonists: same as other HMG-CoA reductase inhibitor, for patients of simultaneously usage of vitamin K antagonists (such as warfarin), at the beginning or gradually increasing the dose of this product may cause the increase of INR (international normalized ratio). Stop using the product or gradually decreasing the dose can lead to decrease in INR. In this case, appropriate INR testing is needed.
3. Combination of gemfibrozil, fenofibrate, other fibrates and lipid-lowering doses (≥1g/day) of niacin with HMG-CoA reductase inhibitors can increase the risk of myopathy, which may be due to that they can cause myopathy when given alone.
4. Antacids: Mixing the product and one antacid containing aluminum hydroxide and magnesium for suspension can reduce the concentration of statin rosuvastatin plasma by 50%. Taking antacids two hours after taking this can further reduce the impact. The clinical significance of this drug interaction has not been studied.
5. Erythromycin: Combination with erythromycin can cause rosuvastatin AUC (0~t) decrease by 20% and Cmax decrease by 30%. This interaction may be due to the gastrointestinal motility caused by increase of erythromycin.
6. Oral administration of contraceptives/hormone replacement therapy (HRT): while using this drug together with oral contraceptives can cause the increase of ethinyl estradiol and norgestrel AUC by 26% and 34%, respectively. When choosing the dose of oral contraceptives, the patients should consider the increase of the plasma concentration of these drugs. Yet there are no pharmacokinetic data on taking rosuvastatin and HRT together. Therefore, we cannot exclude the presence of similar interactions. However, in clinical trials, this kind of combination is widely applied and can be well tolerated by patients.
Product detail pictures:
Related Product Guide:
Our advantages are lower prices,dynamic sales team,specialized QC,strong factories,high quality products and services for Best Price for Fingolimod HCl - Rosuvastatin Calcium CAS 147098-20-2 High Purity – Ruifu , The product will supply to all over the world, such as: Puerto Rico, Angola, Uruguay, If any product meed your demand, remember to feel free to contact us. We're sure your any inquiry or requirement will get prompt attention, high-quality merchandise, preferential prices and cheap freight. Sincerely welcome friends all over the world to call or come to visit, to discuss cooperation for a better future!
-
Renewable Design for Palonosetron Hydrochloride...
-
Hot-selling Canagliflozin INT4 - Benzaldehyde ...
-
Free sample for Tetracaine Hydrochloride - Lev...
-
Special Price for (R)-4-Benzyl-2-oxazolidinone ...
-
Reasonable price (S)-3-Amino-3-phenyl-1-propano...
-
Factory Cheap Hot 3-Chloro-2 4 5-trifluorobenzo...
The goods we received and the sample sales staff display to us have the same quality, it is really a creditable manufacturer.
