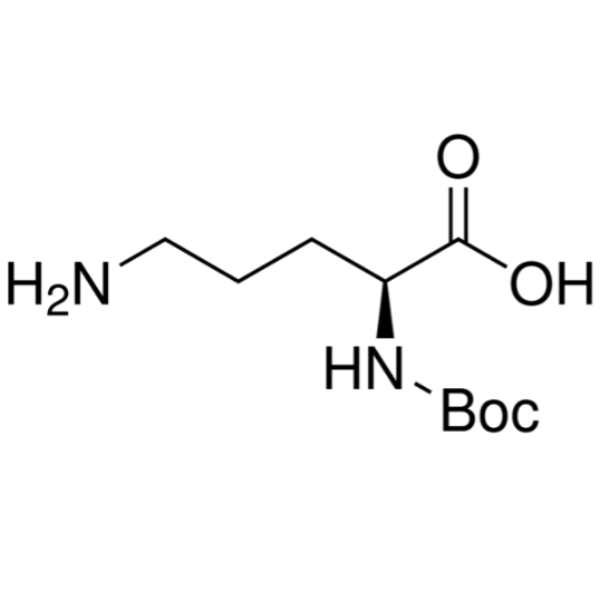
Boc-L-Ornithine CAS 21887-64-9 Boc-Orn-OH Purity >98.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Boc-L-Ornithine (Boc-Orn-OH) (CAS: 21887-64-9) with high quality. Ruifu Chemical supplys a series of amino acids. We can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase Boc-L-Ornithine, Please contact: alvin@ruifuchem.com
| Chemical Name | Boc-L-Ornithine |
| Synonyms | Boc-Orn-OH; Boc-L-Orn-OH; Nα-Boc-L-Ornithine; Nalpha-Boc-L-Ornithine; Nα-tert-Butoxycarbonyl-L-Ornithine; N2-Boc-L-Ornithine; (S)-5-Amino-2-((tert-Butoxycarbonyl)amino)pentanoic Acid; (S)-5-Amino-2-(Boc-Amino)pentanoic Acid |
| Stock Status | In Stock |
| CAS Number | 21887-64-9 |
| Molecular Formula | C10H20N2O4 |
| Molecular Weight | 232.28 g/mol |
| Melting Point | 207.0~208.0℃ |
| Density | 1.135±0.06 g/cm3 |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Category | Boc-Amino Acids |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Powder | White Powder |
| Specific Rotation [α]20/D | +24.0°±2.0° (C=1 in Methanol) |
+23.09° |
| Melting Point | 207.0~208.0℃ | Complies |
| Loss on Drying | <0.50% | 0.22% |
| Residue on Ignition | <0.20% | 0.12% |
| Purity / Analysis Method | >98.0% (HPLC) | 98.76% |
| Infrared Spectrum | Conforms to Structure | Complies |
| NMR Spectrum | Conforms to Structure | Complies |
| Conclusion | The product has been tested & complies with the specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry (2~8℃) warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 43 - May cause sensitization by skin contact
Safety Description S36/37 - Wear suitable protective clothing and gloves.
S60 - This material and its container must be disposed of as hazardous waste.
S37 - Wear suitable gloves.
S24 - Avoid contact with skin.
WGK Germany 3
HS Code 29224999
Boc-L-Ornithine (Boc-Orn-OH) (CAS: 21887-64-9), is a boc-protected analogue of L-Orthinine, a non essential amino acid and intermediate in arginine biosynthesis.
Boc-L-Ornithine can be used in peptide synthesis, reaction type: Boc solid-phase peptide synthesis. Used as an organic synthesis intermediate, pharmaceutical intermediate, biochemical reagent or chemical reagent.
Boc-L-Ornithine can be used to protect L-Ornithine to avoid side reactions. Protecting amino acids is the most basic raw material for synthesizing polypeptides. In order to avoid side reactions, amino groups that do not need to be reacted should be protected in advance so that they do not participate in the reaction during the synthesis process, so as to avoid the generation of by-products.
-
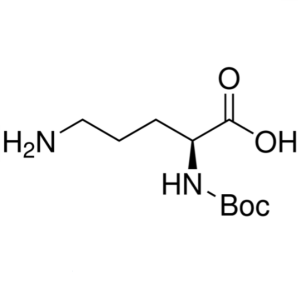
Boc-L-Ornithine CAS 21887-64-9 Boc-Orn-OH Purit...
-
Boc-D-Orn-OH CAS 159877-12-0 Nα-Boc-D-Ornithine...
-
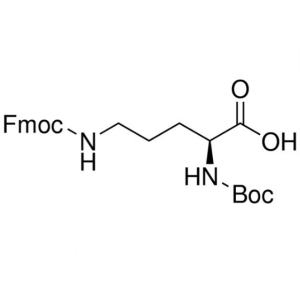
Boc-Orn(Fmoc)-OH CAS 150828-96-9 Nα-Boc-Nδ-Fmoc...
-
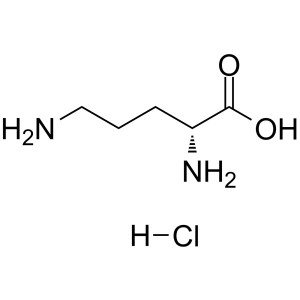
D-Ornithine Monohydrochloride CAS 16682-12-5 As...
-
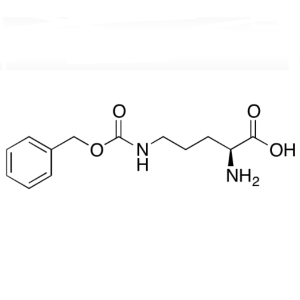
H-Orn(Z)-OH CAS 3304-51-6 N’-Cbz-L-Ornith...
-
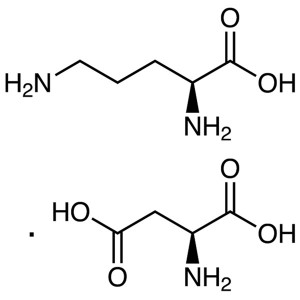
L-Ornithine L-Aspartate CAS 3230-94-2 (L-Orn-L-...
-
Nα-Acetyl-L-Ornithine CAS 6205-08-9 (Ac-Orn-OH)...