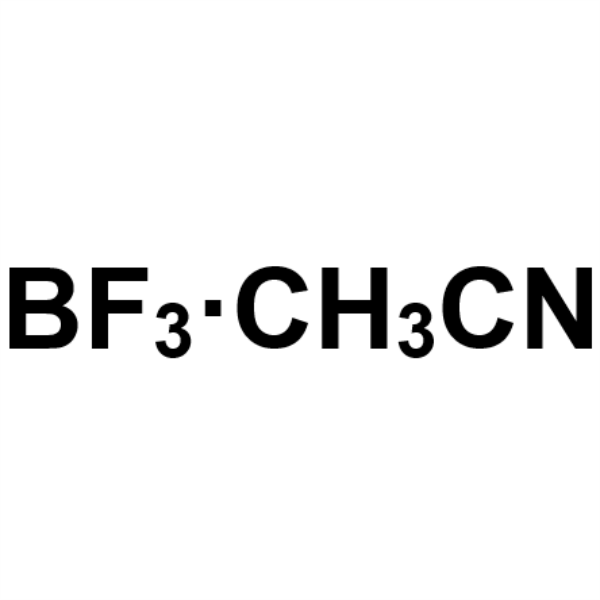Boron Trifluoride Acetonitrile Complex Solution CAS 420-16-6 BF3 ≥19.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Boron Trifluoride Acetonitrile Complex Solution (CAS: 420-16-6) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | Boron Trifluoride Acetonitrile Complex Solution |
| CAS Number | 420-16-6 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C2H3BF3N |
| Molecular Weight | 108.86 |
| Density | 0.87~0.88 g/mL at 20℃ |
| Flash Point | 10℃ |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications | Results |
| Appearance | Colorless or Light Yellow Transparent Liquid | Light Yellow Transparent Liquid |
| BF3 | ≥19.0% (Titration by NaOH 0.1M) | 20.2% |
| Water | ≤0.50% | 0.10% |
| Density (20℃) | 0.88~0.91 g/ml | 0.905 g/ml |
| Iron (Fe) | ≤0.0003% | <0.0003% |
| Proton NMR Spectrum | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, 25kg/Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Keep away from strong direct sunshine; avoid fire; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
1 Characteristics
This product is colorless or light yellow transparent liquid.
2 Identification (should be positive)
The sample was 1.0ml placed in 100 bottle, water was added to the scale and tested on the gas chromatograph. The main peak retention time of the sample should be consistent with that of the acetonitrile control product.
3 Color (≤Y2#)
Take a sample of 10ml, and compare it with the yellow No. 2 standard colourometric solution QM04015-00(Operating procedures for color inspection).
4 Iron ion content (≤3ppm)
Take 1g of this product, and then dilute it with water to make 25ml, check according to QM04008-00 (Operation procedure for determination of iron salt) method, and compare with the control solution made of standard iron solution 0.3ml, not deeper.
5 Moisture: (≤0.5%)
5.1 Reagents and solutions
Karl Fischer's reagent
5.2 Instruments and devices
KF-1 moisture tester
5.3 Operation Methods
Take a dry moisture measurement bottle, weigh an appropriate amount of sample (about 1.0g) into the titration cup, record the corresponding weight, respectively measured twice, according to the moisture measurement operating procedures method to determine the moisture.
5.4 Data Processing
Take the secondary water value and calculate it on average. If the parallel standard deviation of the secondary data is greater than 0.3%, a retest should be conducted
6 Content determination (≥19.0%)
6.1 Instruments and appliances: analytical balance, 100ml stopper triangle bottle, 60ml drop bottle, oven.
6.2 Reagent: Sodium fluoride.
6.3 Determination method: Weigh and analyze about 5g of pure sodium fluoride and place them in two 100ml stopper triangle bottles, respectively, in an oven at 120℃ for about 2 hours (remove the stopper when drying). Remove and cool in the dryer, accurately weigh m1. Using a 60ml drop bottle sample, the sample was weighed by subtractive method of about 1.0g (about 1.2ml) and accurately weighed m. Drop into the triangular bottle, shake well, and place at 30-40℃ for 10min(be sure to plug the bottle). Then put the triangle bottle in the oven at 140℃ for 2 hours, take it out and cool it in the dry, and then accurately weigh m2.
3.4 Calculation formula:
BF3%= (m2-m1)/m ×100%
Where: m1 is sodium fluoride and total amount g of triangle bottle
m2 is the total amount of boron trifluoride, sodium fluoride and triangle bottle g
m is the sample mass g
6.5 Allowable Error
The relative deviation must not exceed 0.3%

Risk Codes R11 - Highly Flammable
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
R34 - Causes burns
R48/23 -
R35 - Causes severe burns
R26 - Very Toxic by inhalation
R21/22 - Harmful in contact with skin and if swallowed.
R14 - Reacts violently with water
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S28 - After contact with skin, wash immediately with plenty of soap-suds.
S16 - Keep away from sources of ignition.
UN IDs UN 2924 3/PG 2
WGK Germany 2
FLUKA BRAND F CODES 10-21
HS Code 2942000000
Boron Trifluoride Acetonitrile Complex Solution (CAS: 420-16-6) is highly soluble in water and soluble in alcohol, ether and other organic solvents. It is mainly used as a catalyst for organic reactions and is an important raw material for the preparation of boron halide, elemental boron, borane, sodium borohydride and other borides. It is the basic raw material for the manufacture of boron hydrogen high-energy fuel and the extraction of isotopes. It can also be used as the curing agent of epoxy resin. It is generally prepared by the reaction of fuming sulfuric acid, boric acid, hydrogen fluoride and acetonitrile. Boron Trifluoride Acetonitrile Complex Solution is a highly active catalyst, which can be used in various organic synthesis reactions, especially in the synthesis of cephalosporin antibacterial drugs. The introduction of boron trifluoride complex can shorten the reaction time of the original product and greatly improve the yield of the original product.
-
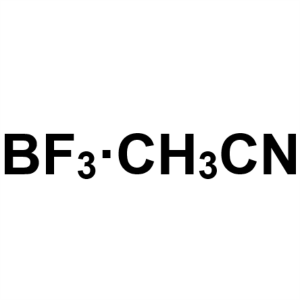
Boron Trifluoride Acetonitrile Complex Solution...
-
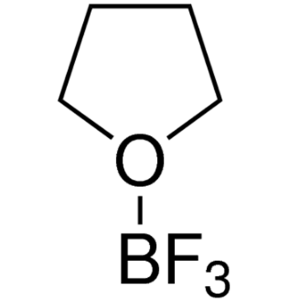
Boron Trifluoride Tetrahydrofuran Complex CAS 4...
-
Boron Trifluoride-Acetic Acid Complex CAS 373-6...
-
Boron Trifluoride-Methanol Solution CAS 373-57-...
-
Borane-2-Methylpyridine Complex CAS 3999-38-0 P...
-
Borane-Pyridine Complex (PYB) CAS 110-51-0 Puri...
-
Borane-Trimethylamine Complex (TMAB) CAS 75-22-...
-
Pyridine Sulfur Trioxide Complex CAS 26412-87-3...
-
Borane-Dimethyl Sulfide Complex 2.0M Solution i...
-
Isopropylmagnesium Chloride Lithium Chloride Co...