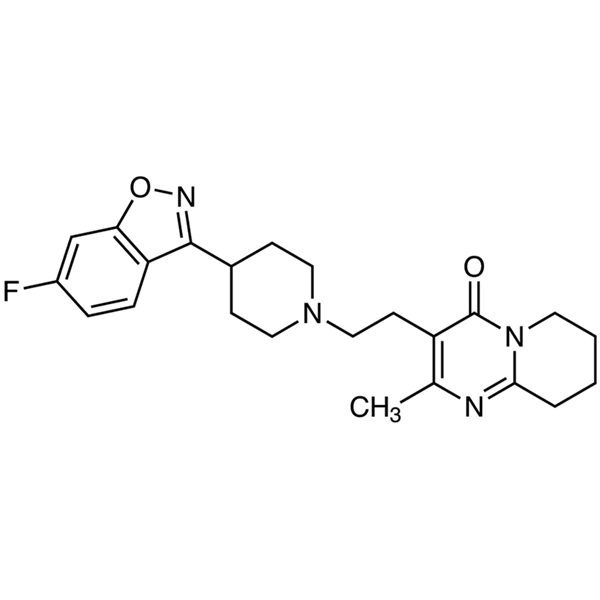
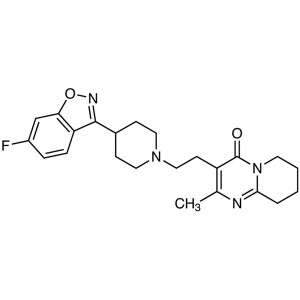
Risperidone CAS 106266-06-2 Assay 98.0~102.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Risperidone (CAS: 106266-06-2) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Risperidone, Please contact: alvin@ruifuchem.com
| Chemical Name | Risperidone |
| Synonyms | 3-[2-[4-(6-Fluoro-1,2-Benzisoxazol-3-yl)-1-Piperidinyl]ethyl]-6,7,8,9-Tetrahydro-2-Methyl-4H-Pyrido[1,2-a]pyrimidin-4-one; Risperdal; Risperidal; Risperdal Consta; Rispolept; Risperin; Rispolin; Sequinan; Risperdal M-Tab |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 106266-06-2 |
| Molecular Formula | C23H27FN4O2 |
| Molecular Weight | 410.49 g/mol |
| Melting Point | 170.0 to 174.0℃ |
| Density | 1.38±0.10 g/cm3 |
| Hazard Class | 6.1; Poison |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Powder | Complies |
| Identification A | Infrared Absorption | Complies |
| Identification B | Retention Time: Corresponds to Reference Substance | Complies |
| Loss on Drying | <0.50% | 0.12% |
| Residue on Ignition | <0.10% | 0.07% |
| Heavy Metals | <0.001% | <0.001% |
| Related Compounds | ||
| E-Oxime | <0.20% | <0.20% |
| Z-Oxime | <0.20% | <0.20% |
| 9-Hydroxyrisperidone | <0.20% | <0.20% |
| 5-Fluororisperidone | <0.20% | <0.20% |
| 6-Methylrisperidone | <0.20% | <0.20% |
| Any Unknown Impurity | <0.10% | <0.10% |
| Total Impurities | <0.30% | <0.30% |
| Assay | 98.0~102.0% (Calculated on the Dried Basis) | 100.52% |
| Conclusion | The product has been tested and complies with the USP 35 Standard | |
Package: Bottle, 1kg/Tin, 5kg/Tin, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Risperidone
C23H27FN4O2 410.48
4H-Pyrido[1,2-a]pyrimidin-4-one, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-.
3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one [106266-06-2].
» Risperidone contains not less than 98.0 percent and not more than 102.0 percent of C23H27FN4O2, calculated on the dried basis.
Packaging and storage- Preserve in well-closed containers. Store at room temperature.
USP Reference standards <11>-
USP Risperidone RS
3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidino]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one 410.48 CAS-106266-06-2
USP Risperidone System Suitability Mixture RS
Contains risperidone and about 0.2% of each of the following:
Z-Oxime-3-[2-[4-[(Z)-(2,4-difluorophenyl)(hydroxyimino)methyl]piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one.
9-Hydroxyrisperidone-(6RS)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2,6-dimethyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one.
6-Methylrisperidone-(6RS)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2,6-dimethyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one.
[Note-This mixture contains risperidone, Z-oxime, 9-hydroxyrisperidone, and 6-methylrisperidone.]
Identification-
A: Infrared Absorption <197K>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Loss on drying <731>- Dry in vacuum at 80 for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition <281>: not more than 0.1%, a 2.0-g test specimen being used.
Heavy metals, Method II <231>: 0.001%.
Related compounds-
Buffer solution, Solution A, Solution B, Mobile phase, Diluent, System suitability solution, and Chromatographic system-Prepare as directed in the Assay.
Standard solution-Use the Standard preparation, prepared as directed in the Assay.
Test solution-Use the Assay preparation.
Procedure-Inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, and record the chromatogram. Identify the impurities using the relative retention times given in Table 1, and measure the peak responses. Calculate the percentage of each risperidone related compound in the portion of Risperidone taken by the formula:
100(CS /CU)(rU / rS)(1/F)
in which CS and CU are the concentrations, in mg per mL, of risperidone in the Standard solution and the Test solution respectively; rU is the peak area of each impurity obtained from the Test solution; rS is the peak area of risperidone obtained from the Standard solution; and F is the relative response factor for each impurity relative to risperidone. In addition to not exceeding the limits in Table 1, not more than 0.10% of any unknown impurity (use F value of 1.0) is found and not more than 0.30% of the total impurities is found. Disregard the impurity peaks that are less than 0.05%.
Table 1
Related Compound Relative Retention Time (RRT) Relative Response Factor (F) Limit (%)
E-oxime1 0.60 1.0 NMT 0.20
Z-oxime2 0.67 0.63 NMT 0.20
9-hydroxyrisperidone3 0.76 0.92 NMT 0.20
5-fluororisperidone4 0.94 1.0 NMT 0.20
Risperidone 1.0 1.0 -
6-methylrisperidone5 1.2 0.95 NMT 0.20
1 3-[2-[4-[(E)-(2,4-Difluorophenyl)(hydroxyimino)methyl]piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
2 3-[2-[4-[(Z)-(2,4-Difluorophenyl)(hydroxyimino)methyl]piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
3 (9RS)-3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
4 3-[2-[4-(5-Fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
5 (6RS)-3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2,6-dimethyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
Assay-
Buffer solution- Dissolve 15.4 g of ammonium acetate in 1 L of water. Adjust with 10% acetic acid to a pH of 6.5, and mix.
Solution A-Mix 100 mL of Buffer solution with 150 mL of methanol in a 1000-mL volumetric flask, and dilute with water to volume.
Solution B- Mix 100 mL of Buffer solution with 850 mL of methanol in a 1000-mL volumetric flask, and dilute with water to volume.
Mobile phase-Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography 621).
Diluent-Mix 100 mL of Buffer solution with 900 mL of water and 1000 mL of methanol.
System suitability solution-Prepare a 1 mg per mL solution of USP Risperidone System Suitability Mixture RS in Diluent.
Standard preparation-Dissolve an accurately weighed quantity of USP Risperidone RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 1.0 mg per mL.
Assay preparation-Dissolve an accurately weighed quantity of Risperidone in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a concentration of about 1.0 mg per mL.
Chromatographic system (see Chromatography <621>)- The liquid chromatograph is equipped with a 275-nm detector and a 4.6-mm × 10-cm column that contains 3-µm packing L1. The flow rate is about 1.5 mL per minute. The column temperature is maintained at 35. The chromatograph is programmed as follows.
Time (minutes) Solution A (%) Solution B (%) Elution
0–1 70 30 isocratic
1–20 70®5 30®95 linear gradient
20–25 5 95 isocratic
25–27 5®70 95®30 linear gradient
27–35 70 30 re-equilbrationInject the System suitability solution. Record the peak responses as directed for Procedure, and identify the peaks due to Z-oxime, 9-hydroxyrisperidone, 6-methylrisperidone, and risperidone using the relative retention times (RRT) from Table 1; the resolution, R, between Z-oxime and 9-hydroxyrisperidone is not less than 2.8; the tailing factor for risperidone is not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0% for the risperidone peak.
Procedure- Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, and measure the responses for the risperidone peak. Calculate the quantity, in percent of C23H27FN4O2, in the portion of Risperidone taken by the formula:
100(CS /CU)(rU / rS)
in which CS and CU are the concentrations, in mg per mL, of risperidone in the Standard preparation and the Assay preparation, respectively; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes R25 - Toxic if swallowed
R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed.
R11 - Highly Flammable
Safety Description S28 - After contact with skin, wash immediately with plenty of soap-suds.
S36 - Wear suitable protective clothing.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37 - Wear suitable protective clothing and gloves.
S16 - Keep away from sources of ignition.
UN IDs 3249
WGK Germany 3
RTECS UV1164800
HS Code 2934999099
Hazard Class 6.1(a)
Packing Group II
Risperidone (CAS: 106266-06-2) has antagonism effect on 5HT2 receptors and D2 receptors. Risperidone is used for acute and chronic schizophrenia. Antipsychotic drugs. Risperidone is used for the treatment of acute and chronic schizophrenia and other various psychotic state obvious positive symptoms (such as hallucinations, illusions, thought disorder, hostility, suspicion) and significant negative symptoms (such as unresponsiveness, emotional apathy and social apathy, of few words). Also it can alleviate affective symptoms associated with schizophrenia (such as: depression, guilt, anxiety). For patients whom Risperidone is effective to for acute treatment ,in the maintenance phase of treatment, the Risperidone can continue to play its clinical efficacy. Risperidone, therefore, has therapeutic action on both positive and negative symptoms of schizophrenia and produces significantly fewer side effects especially extrapyramidal symptoms compared with commonly used pure D2 antagonist antipsychotics. It also has potential for management of alcohol withdrawal and cocaine addiction.