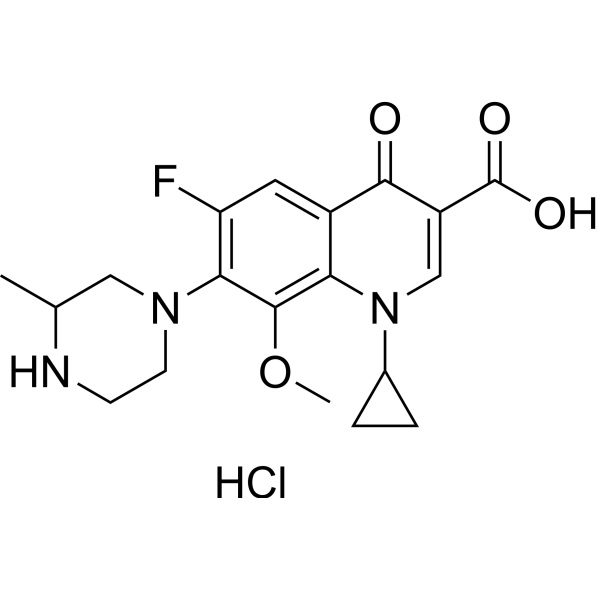
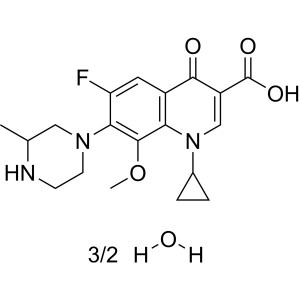
Gatifloxacin Hydrochloride CAS 160738-57-8 Purity >98.5% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Gatifloxacin Hydrochloride (CAS: 160738-57-8) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Gatifloxacin Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Gatifloxacin Hydrochloride |
| Synonyms | Gatifloxacin HCl; Gatifloxacin Base; 1-Cyclopropyl-6-Fluoro-1,4-Dihydro-8-Methoxy-7-(3-Methylpiperazin-1-yl)-4-Oxo-3-Quinolinecarboxylic Acid Hydrochloride; 1-Cyclopropyl-6-Fluoro-8-Methoxy-7-(3-Methylpiperazin-1-yl)-4-Oxo-1,4-Dihydroquinoline-3-Carboxylic Acid Hydrochloride; 1-Cyclopropyl-6-Fluoro-8-Methoxy-7-(3-Methyl-Piperazin-1-yl)- 4-Oxo-1,4-Dihydro-Quinoline-3-Carboxylic Acid Hydrochloride |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 160738-57-8 |
| Molecular Formula | C19H22FN3O4·HCl |
| Molecular Weight | 411.86 g/mol |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | Off-White to Light Yellow Crystalline Powder | Complies |
| Purity | >98.5% (HPLC) | 99.55% |
| Moisture | <1.00% | 0.45% |
| Residue on Ignition | <0.20% | 0.06% |
| Heavy Metals (Pb) | ≤20ppm | <10ppm |
| Related Substances | <1.00% | 0.45% |
| Infrared Spectrum | Consistent with Structure | Complies |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Clarity & Color | Meet the Requirements | Meet the Requirements |
| Residual Solvent | Meet the Requirements | Meet the Requirements |
| Conclusion | The product has been tested and complies with the given specifications | |
| Shelf life | 24 Months if Stored Properly | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum lined with double-layer plastic bag, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture, avoid fire and heat.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes
36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S27 - Take off immediately all contaminated clothing.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
Gatifloxacin Hydrochloride (CAS: 160738-57-8) is a novel orally-active fluoroquinolone antibiotic with a potent and broad spectrum of activity against gram-positive (S.pneumonia, S.aureus) and gram-negative bacteria. It shows good activity against strains resistant to established antituberculosis agents. Gatifloxacin was marketed for the treatment of respiratory tract and urinary infections, particularly the treatment of certain community-acquired infections (such as bronchitis, pneumonia and common sexually-transmitted diseases). Comparative studies with ciprofloxacin, ofloxacin and sparfloxacin against fluoroquinolone-resistant organisms indicated a better or equivalent effectiveness with less phototoxic adverse effects.
For the treatment of moderate or more infectious diseases caused by sensitive strains:
1. Acute attack of chronic bronchitis: caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis or Staphylococcus aureus.
2. Acute sinusitis: caused by Streptococcus pneumoniae and Haemophilus influenzae.
3. Community acquired pneumonia: caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis, Staphylococcus aureus, Chlamydia pneumophila, Mycoplasma pneumophila, Legionella pneumophila, etc.
4. Simple or complex urinary tract infection (cystitis): caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, etc.
5. Pyelonephritis: caused by Escherichia coli, etc.
6. Simple urethral and cervical gonorrhea: caused by Neisseria gonorrhoeae.
7. Acute simple rectal infection in women: caused by Neisseria gonorrhoeae.