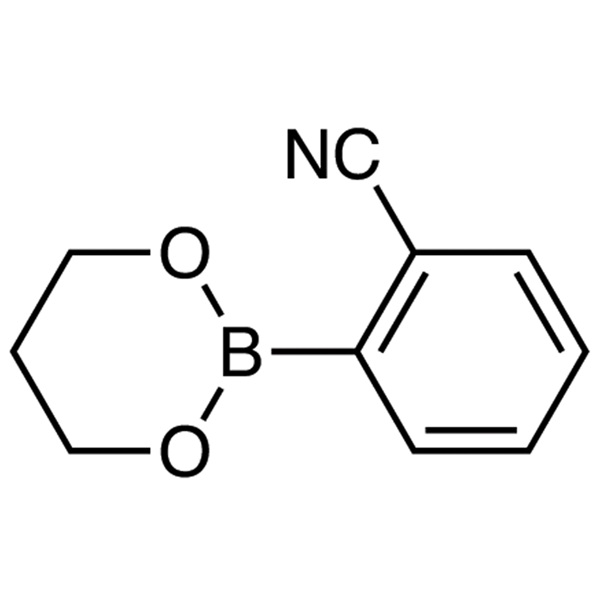
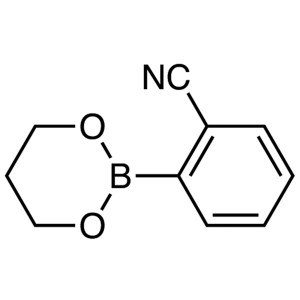
2-Cyanophenylboronic Acid 1,3-Propanediol Ester CAS 172732-52-4 Perampanel Intermediate Purity >99.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of 2-Cyanophenylboronic Acid 1,3-Propanediol Ester (CAS: 172732-52-4) with high quality, intermediate of Perampanel (CAS: 380917-97-5). Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Perampanel intermediates, Please contact: alvin@ruifuchem.com
| Chemical Name | 2-Cyanophenylboronic Acid 1,3-Propanediol Ester |
| Synonyms | 2-Cyanobenzeneboronic Acid 1,3-Propanediol Ester; 2-(1,3,2-Dioxaborinan-2-yl)benzonitrile |
| CAS Number | 172732-52-4 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H10BNO2 |
| Molecular Weight | 187.01 |
| Melting Point | 46.0~51.0℃ (lit.) |
| Density | 1.13±0.10 g/cm3 |
| Solubility | Soluble in Methanol |
| Stability | Hygroscopic |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Light Yellow Powder to Crystal |
| Purity / Analysis Method | >99.0% (HPLC) |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Single Impurity | <0.50% |
| Total Impurities | <1.00% |
| Heavy Metals (as Pb) | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Perampanel (CAS: 380917-97-5) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.

Hazard Symbols Xi - Irritant
Risk Codes
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
R36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
UN IDs 3439
WGK Germany 3
TSCA No
Hazard Note Irritant
2-Cyanophenylboronic Acid 1,3-Propanediol Ester (CAS: 172732-52-4) is used in the synthesis of Perampanel (CAS: 380917-97-5), an AMPA receptor antagonist. Perampanel (licensed in 2012) is a third- generation AED known with the proprietary brand name of Fycompa® (Eisai, Hatfield) in the UK and Banzel® (Eisai, Hatfield) in the USA. In October 2012, the US FDA approved perampanel for the treatment of partial onset seizures in epileptic patients who are at least 12 years old. Perampanel is the first AMPA receptor antagonist to receive FDA approval as an AED. AMPA glutamate receptors are found primarily on postsynaptic neurons in the brain. As a selective, noncompetitive antagonist of AMPA, parampanel prevents ion channel opening and reduces propagation of action potential. Perampanel was approved by European EMA and US FDA in July and October 2012 respectively, it is used for adjuvant treatment of patients with partial seizures over 12 years old with or without secondary systemic seizures. On June 22, 2015, Eisai announced that the US FDA has approved the expansion of the indication of Pirenparanide hydrate (Fycompa) as an adjuvant treatment for primary comprehensive tonic-clonic seizures in patients with epilepsy aged 12 and above.