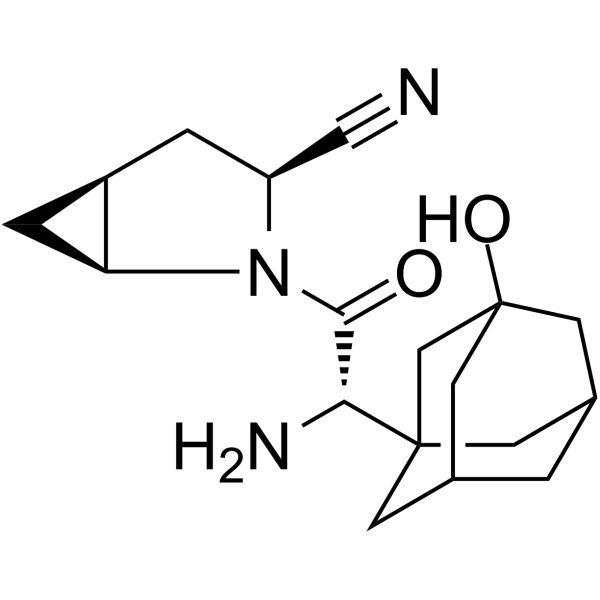
CAS 361442-04-8 Purity >99.0% (HPLC) API
Ruifu Chemical Supply Related Intermediates
CAS 361442-04-8
CAS 945667-22-1
(R)-1-Boc-3-Aminopiperidine CAS 188111-79-7
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 334618-23-4
Boc-3-Hydroxy-1-Adamantyl-D-Glycine CAS 361442-00-4
(1S,3S,5S)-3-(Aminocarbonyl)-2-Azabicyclo [3.1.0]hexane-2-Carboxylic Acid tert-Butyl Ester CAS 361440-67-7
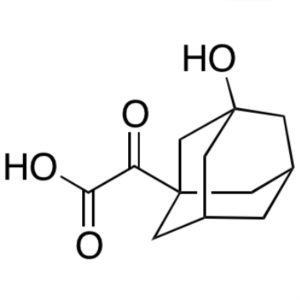
2-(3-Hydroxy-1-Adamantyl)-2-Oxoacetic Acid CAS 709031-28-7
| Synonyms | BMS-477118; Onglyza; (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-Hydroxy-1-Adamantyl)acetyl]-2-Azabicyclo[3.1.0]hexane-3-Carbonitrile; (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-Hydroxytricyclo [3.3.1.13,7]dec-1-yl)acetyl]-2-Azabicyclo[3.1.0]hexane-3-Carbonitrile |
| CAS Number | 361442-04-8 |
| CAT Number | RF-PI1991 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C18H25N3O2 |
| Molecular Weight | 315.41 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White Powder |
| Odor | Characteristic |
| identification | A: IR B: HPLC Retention Time |
| Purity / Analysis Method | >99.0% (HPLC) |
| Clarity of Water Solution | Transparent, Colorless, None Suspended Matters |
| Sieve Analysis | 100% Pass 80 Mesh |
| Melting Point | 202.0~212.0℃ |
| Loss on Drying | <0.50% |
| Sulphated Ash | <0.20% |
| Specific Rotation | +174.0°~+186.0° |
| Single Impurity | <0.50% |
| Total Impurities | <1.00% |
| Heavy Metals | <10ppm |
| Arsenic (As) | <2.0ppm |
| Lead (Pb) | <1.0ppm |
| Cadmium (Cd) | <1.0ppm |
| Residual Solvents | <100ppm |
| Residual Pesticide | Negative |
| Microbiology | |
| Total Plate Count | <1000cfu/g |
| Yeast & Mold | <100cfu/g |
| E. Coli | Negative |
| S. Aureus | Negative |
| Salmonella | Negative |
| Shelf Life | 24 Months From Manufacture Date if Stored Properly |
| Test Standard | Enterprise Standard |
| Usage | API; Type 2 Diabetes Mellitus |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


(BMS-477118) (CAS: 361442-04-8) is a potent, selective, reversible, competitive and orally active dipeptidyl peptidase-4 (DPP-4) (Ki = 0.6-1.3 nM) inhibitor. BMS-477118 has the peotential for type 2 diabetes mellitus research. BMS-477118 is a type 2 diabetes drug that can stimulate the pancreas to produce more insulin after the meal. It was reached by the cooperation of AstraZeneca and Bristol-Myers Squibb Company and belongs to DPP-IV inhibitor. BMS-477118 plays the role through inhibiting GLP-l degradation. GLP-I is the hormones naturally produced in the intestine after taking food. It can regulate the secretion of insulin and strengthen the utilization of glucose in the peripheral tissues. The single medication of BMS-477118 can improve blood glucose control and the combined medication of BMS-477118 with Metformin, Sulfonylurea and Thiazolidinediones can enhance curative effect. It leads to low risk of hypoglycemia and its adverse reactions are similar to placebo, showing better tolerance. On July 31, 2009, BMS-477118 tablets (Onglyza), a new drug of type 2 diabetes, jointly researched and developed AstraZeneca and Bristol-Myers Squibb was approved by the US FDA. It can be taken once a day to treat type 2 diabetes combined with controlling Diet and exercise.
-
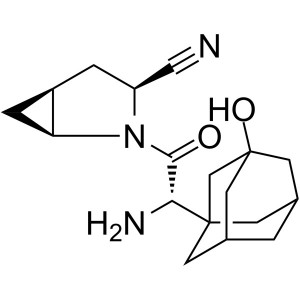
CAS 361442-04-8 Purity >99.0% (HPLC) API
-
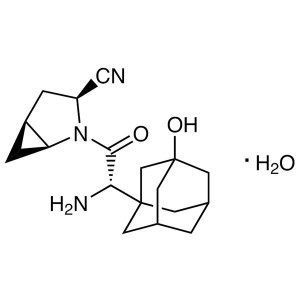
CAS 945667-22-1 Purity >99.0% (HPLC) API
-
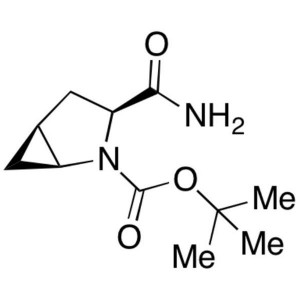
CAS 361440-67-7 Purity >98.5% (HPLC) Factory
-
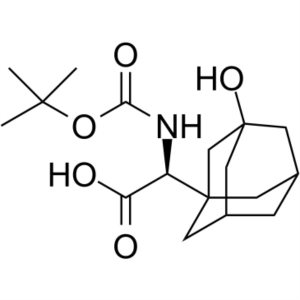
Boc-3-Hydroxy-1-Adamantyl-D-Glycine CAS 361442-...
-
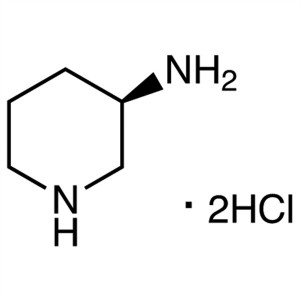
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 3...
-
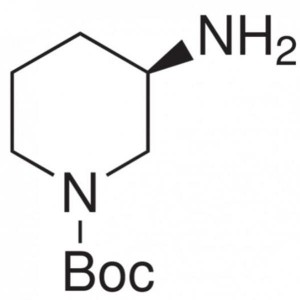
(R)-1-Boc-3-Aminopiperidine CAS 188111-79-7 Pur...
-
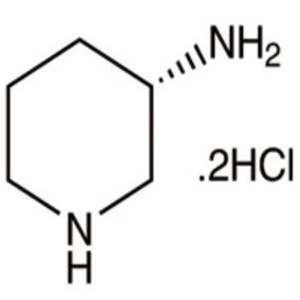
(S)-(+)-3-Aminopiperidine Dihydrochloride CAS 3...
-
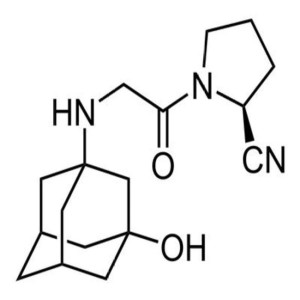
Vildagliptin CAS 274901-16-5 Assay 98.0%~102.0%...
-
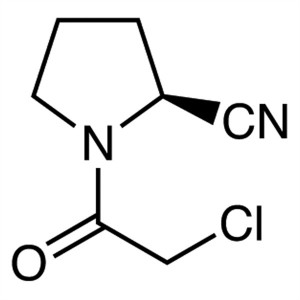
(2S)-1-(Chloroacetyl)-2-Pyrrolidinecarbonitrile...
-
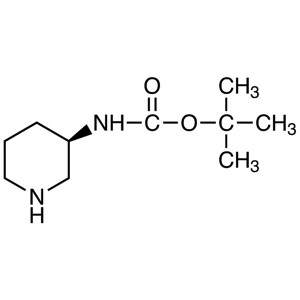
(R)-3-(Boc-Amino)piperidine CAS 309956-78-3 Lin...
-
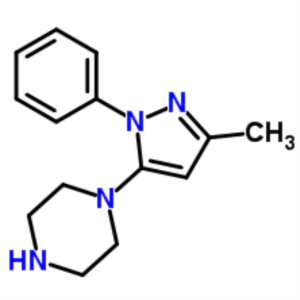
1-(3-Methyl-1-Phenyl-5-Pyrazolyl)piperazine CAS...
-
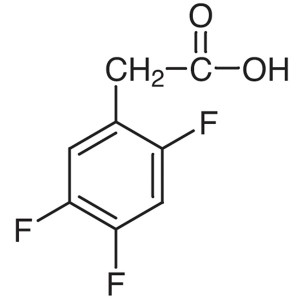
2,4,5-Trifluorophenylacetic Acid CAS 209995-38-...
-
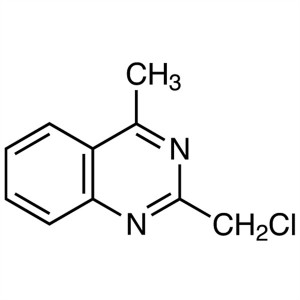
2-(Chloromethyl)-4-Methylquinazoline CAS 109113...
-
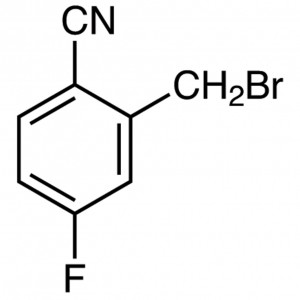
2-Cyano-5-Flurobenzyl Bromide CAS 421552-12-7 P...
-
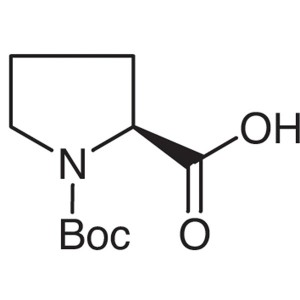
Boc-L-Proline CAS 15761-39-4 (Boc-Pro-OH) Purit...
-
2-(3-Hydroxy-1-Adamantyl)-2-Oxoacetic Acid CAS ...