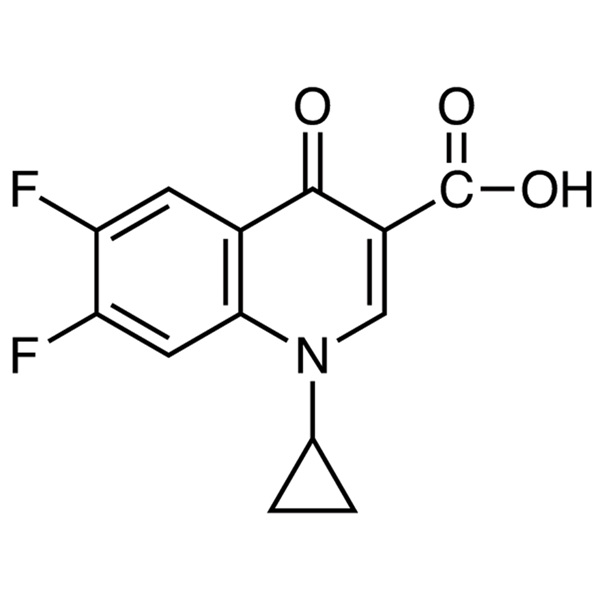
Moxifloxacin Difluoro Acid Impurity CAS 93107-30-3 Purity >99.0% (HPLC)
Ruifu Chemical is the leading manufacturer of 1-Cyclopropyl-6,7-Difluoro-1,4-Dihydro-4-Oxoquinoline-3-Carboxylic Acid (CAS: 93107-30-3) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase, Please contact: alvin@ruifuchem.com
| Chemical Name | 1-Cyclopropyl-6,7-Difluoro-1,4-Dihydro-4-Oxoquinoline-3-Carboxylic Acid |
| Synonyms | 1-Cyclopropyl-6,7-Difluoro-4-Oxo-1,4-Dihydroquinoline-3-Carboxylic Acid; Moxifloxacin Difluoro Acid Impurity; Moxifloxacin Impurity 24 |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 93107-30-3 |
| Molecular Formula | C13H9F2NO3 |
| Molecular Weight | 265.22 |
| Melting Point | 289°C(lit.) |
| Density | 1.630±0.06 g/cm3 |
| COA & MSDS | Available |
| Sample | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | Off-White to Cream Color Powder | Conforms |
| Purity / Analysis Method | >99.0% (HPLC) | 99.78% |
| Main Single Impurity | <0.50% | 0.20% |
| Loss on Drying | <0.50% | 0.06% |
| Residue on Ignition | <0.20% | 0.03% |
| 1H NMR Spectrum | Conforms to Structure | Conforms |
| LC-MS for Identification | Conforms | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xn - Harmful
Risk Codes R62 - Possible risk of impaired fertility
R52/53 - Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Description S22 - Do not breathe dust.
S36/37 - Wear suitable protective clothing and gloves.
S61 - Avoid release to the environment. Refer to special instructions / safety data sheets.
1-Cyclopropyl-6,7-Difluoro-1,4-Dihydro-4-Oxoquinoline-3-Carboxylic Acid (Moxifloxacin Difluoro Acid Impurity) (CAS: 93107-30-3) is an Impurity of Moxifloxacin. Moxifloxacin is a fourth-generation synthetic fluoroquinolone antibacterial agent developed by Bayer AG (initially called BAY 12-8039), used to treat a number of infections, including: respiratory tract infections, cellulitis, anthrax, intraabdominal infections, endocarditis, meningitis, and tuberculosis. It is marketed worldwide (as the hydrochloride) under the brand names Avelox, Avalox, and Avelon for oral treatment. In most countries, the drug is also available in parenteral form for intravenous infusion.