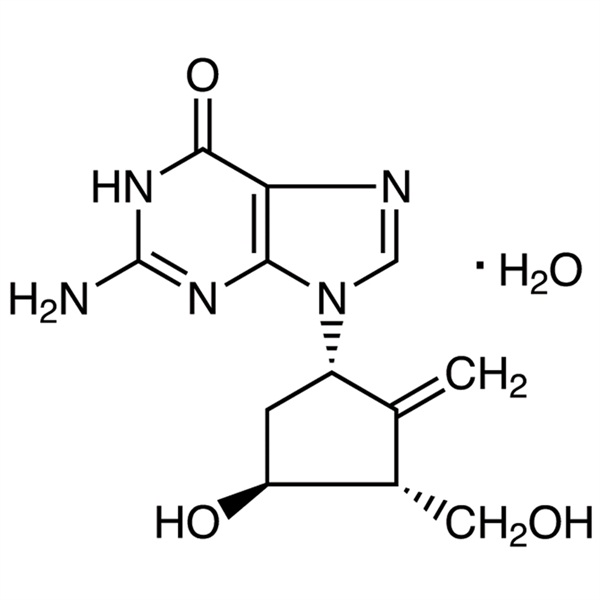
Entecavir Monohydrate CAS 209216-23-9 Assay 98.0%~102.0% API USP EP Standard Antiviral Hepatitis B Infection
Ruifu Chemical is the leading supplier of Entecavir Monohydrate (CAS: 209216-23-9) with high quality, can meet the USP / EP standard, used in the treatment of Hepatitis B Infection.
Ruifu has been supplying APIs and pharmaceutical intermediates more than 15 years.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Entecavir Monohydrate, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Entecavir Monohydrate |
| Synonyms | Entecavir Hydrate; 9-[(1S,3R,4S)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine Monohydrate |
| CAS Number | 209216-23-9 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C12H17N5O4 |
| Molecular Weight | 295.3 |
| Melting Point | >220℃ |
| Density | 1.81 |
| Shipping Condition | Shipped Under Ambient Temperature |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Almost White Crystalline Powder |
| Solubility | Freely Soluble in Dimethylformamide, Slightly Soluble in Water, Ethanol and Methanol |
| Identification IR | Spectrum of the sample corresponds to that of reference standard |
| Identification HPLC | The retention time of the test sample corresponds to that of the reference standard |
| Water Content (by K.F) | 5.5%~6.5% |
| Specific Optical Rotation | +24.0° to +30.0° (DMF: MeOH=1:1 C=1%) (on anhydrous substance) |
| Residue on Ignition | ≤0.10% |
| Related Substances | |
| Furoentecavir | ≤0.10% |
| Entecavir 1-Epimer | ≤0.10% |
| Entecavir 3-Epimer | ≤0.10% |
| 8-Hydroxy-Entecavir | ≤0.10% |
| Entecavir 4-Epimer | ≤0.10% |
| 8-Methoxy Entecavir | ≤0.10% |
| 4-Dimethylsilyl Entecavir | ≤0.10% |
| Entecavir Related Compound A | ≤0.10% |
| Any Unspecified Impurity | ≤0.10% |
| Total Impurities | ≤0.30% |
| Residual Solvents | |
| Methanol | ≤600ppm |
| Dichloromethane | ≤300ppm |
| Ethyl Acetate | ≤1000ppm |
| Tetrahydrofuran | ≤720ppm |
| Toluene | ≤890ppm |
| Benzyl Chloride | ≤1000ppm |
| Benzyl Alcohol | ≤1000ppm |
| Microbial Limit | |
| Total Aerobic Counts | ≤100cfu/g |
| Yeasts and Molds | ≤10cfu/g |
| Escherichia Coli | Should Not Detected |
| Heavy Metals | ≤10ppm |
| Assay | 98.0%~102.0% (HPLC; on the anhydrous basis) |
| Particle Size | 95% of the particles should be within 125μm |
| Test Standard | Enterprise Standard; United States Pharmacopoeia (USP) Standard |
| Usage | Antiviral drug used in the treatment of Hepatitis B Infection |
Package: Bottle, Aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2-15℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.


| Safety Description | 24/25 - Avoid contact with skin and eyes. |
| HS Code | 2933990099 |
Entecavir Monohydrate (CAS 209216-23-9) is an oral antiviral drug used in the treatment of Hepatitis B Virus (HBV) infection. Entecavir Monohydrate is a nucleoside analog (more specifically, a guanine analogue) that inhibits reverse transcription, DNA replication and transcription in the vira. It is a new kind of cyclopentyl acyl guanosine anti-Hepatitis B virus drugs with its pharmacological effects similar as Entecavir. It is clinically applied to the treatment of adult Chronic Hepatitis B in which there is active viral replication, increased serum transaminase ALT or active lesions showed from liver histology. Entecavir Monohydrate is a potent and selective inhibitor of HBV, with an EC50 of 3.75 nM in HepG2 cell. Entecavir is a reverse transcriptase inhibitor. It prevents the Hepatitis B Virus (HBV) from multiplying and reduces the amount of virus in the body.
Entecavir
C12H15N5O3·H2O 295.29
6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-, monohydrate;
9-[(1S,3R,4S)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine monohydrate [209216-23-9]; UNII: 5968Y6H45M.
Anhydrous 277.28
DEFINITION
Entecavir is a monohydrate and contains NLT 98% and NMT 102% of Entecavir (C12H15N5O3), calculated on the anhydrous basis.
IDENTIFICATION
Change to read:
• A. SPECTROSCOPIC IDENTIFICATION TESTS <197>, Infrared Spectroscopy: 197A or 197K (CN 1-May-2020)
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• PROCEDURE
Solution A: Acetonitrile and water (3:97)
Solution B: Acetonitrile
Mobile phase: See Table 1. [NOTE- The gradient elution times are established on an HPLC system with a dwell volume of approximately 1.0 mL.]
Table 1 Time (min) Solution A (%) Solution B (%)
0 100 0
8 100 0
50 77 23
75 17 83
90 100 0
100 100 0
System suitability stock solution: 1.0 mg/mL of USP Entecavir System Suitability Mixture RS in methanol
System suitability solution: 0.2 mg/mL of USP Entecavir System Suitability Mixture RS in Solution A from System suitability stock solution
Standard stock solution: 1.0 mg/mL of USP Entecavir Monohydrate RS in methanol. Sonicate as needed.
Standard solution: 0.2 mg/mL of USP Entecavir Monohydrate RS in Solution A from the Standard stock solution
Sample stock solution: 1.0 mg/mL of Entecavir in methanol. Sonicate as needed.
Sample solution: 0.2 mg/mL of Entecavir in Solution A from Sample stock solution
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[ NOTE- See Table 2 for the relative retention times of the components in the System suitability solution.]
Suitability requirements
Resolution: NLT 3.5 between entecavir 1-epimer and entecavir; NLT 2.0 between entecavir and 8-hydroxy entecavir, System
suitability solution
Tailing factor: 0.8-1.5 for entecavir, System suitability solution
Relative standard deviation: NMT 1.5%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of entecavir (C12H15N5O3) in the portion of Entecavir taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of entecavir from the Sample solution
rs = peak response of entecavir from the Standard solution
Cs = concentration of USP Entecavir Monohydrate RS in the Standard solution (mg/mL)
Cu = concentration of Entecavir in the Sample solution (mg/mL)
Acceptance criteria: 98%-102% on the anhydrous basis
IMPURITIES
• ORGANIC IMPURITIES
Solution A, Solution B, Mobile phase, System suitability stock solution, System suitability solution, Sample stock solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard stock solution: Use the Standard solution from the Assay.
Standard solution: 0.2 µg/mL of USP Entecavir Monohydrate RS in Solution A from the Standard stock solution
System suitability
Samples: System suitability solution and Standard solution
[ NOTE- See Table 2 for relative retention times of the components in the System suitability solution.]
Suitability requirements
Resolution: NLT 3.5 between entecavir 1-epimer and entecavir; NLT 2.0 between entecavir and 8-hydroxy entecavir, System suitability solution
Tailing factor: 0.8-1.5 for entecavir, System suitability solution
Relative standard deviation: NMT 10.0%, Standard solution
Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of each impurity in the portion of Entecavir taken:
Result = (ru /rs ) × (Cs /Cu ) × (1/F) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of entecavir from the Standard solution
Cs = concentration of USP Entecavir Monohydrate RS in the Standard solution (mg/mL)
Cu = concentration of Entecavir in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2. Disregard any peak less than 0.05%.
Table 2
Name Relative Retention Time Relative Response Factor Acceptance Criteria, NMT (%)
Furoentecavir a 0.73 1.0 0.1
Entecavir 1-epimer b 0.93 1.0 0.1
Entecavir 3-epimer c 0.96 1.0 0.1
Entecavir 1.0 - -
8-Hydroxy entecavir d 1.03 0.67 0.1
Entecavir 4-epimer e 1.08 1.0 0.1
8-Methoxy entecavir f 1.27 0.67 0.1
4-Dimethylsilyl entecavir g 1.84 1.0 0.1
Entecavir related compound A 3.41 - -
Any unspecified impurity - 1.0 0.1
Total impurities i - - 0.3
a 9-[(3aS,4S,6S,6aR)-3a,6-Dihydroxyhexahydro-1H-cyclopenta[c]furan-4-yl]guanine.
b 9-[(1R,3R,4S)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
c 9-[(1S,3S,4S)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
d 8-Hydroxy-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
e 9-[(1S,3R,4R)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
f 8-Methoxy-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
g 9-[(1S,3R,4S)-4-Hydroxydimethylsilyl-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
h For information only; quantitated in the test for Limit of Entecavir Related Compound A.
i Includes the sum of all the impurities found in the tests for Limit of Entecavir Related Compound A and Organic Impurities.
• LIMIT OF ENTECAVIR RELATED COMPOUND A
Solution A: 0.1% (v/v) trifluoroacetic acid in water
Solution B: 0.1% (v/v) trifluoroacetic acid in acetonitrile
Mobile phase: See Table 3. [ NOTE- The gradient elution times are established on an HPLC system with a dwell volume of
approximately 1.0 mL.]
Table 3
Time (min) Solution A (%) Solution B (%)
0 65 35
8 53 47
8.1 65 35
11 65 35
Standard solution: 2 µg/mL of USP Entecavir Related Compound A RS in methanol
Sample solution: 1.0 mg/mL of Entecavir in methanol. Sonicate as needed.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 5-cm; 5-µm packing L1
Temperatures
Autosampler: 4°
Column: 30°
Flow rate: 2 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: 0.8-1.5
Relative standard deviation: NMT 3.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of entecavir related compound A in the portion of Entecavir taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of entecavir related compound A from the Sample solution
rs = peak response of entecavir related compound A from the Standard solution
Cs = concentration of USP Entecavir Related Compound A RS in the Standard solution (mg/mL)
Cu = concentration of Entecavir in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.1%
SPECIFIC TESTS
• WATER DETERMINATION <921>, Method I, Method Ic: 5.5%-7.0%
• OPTICAL ROTATION <781S>, Procedures, Specific Rotation
Sample solution: 10 mg/mL of Entecavir in a mixture of dimethylformamide and methanol (50:50)
Acceptance criteria: +24° to +30°
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in well-closed containers, protected from light. Store at room temperature.
• USP REFERENCE STANDARDS <11>
USP Entecavir Monohydrate RS
USP Entecavir Related Compound A RS
3-Benzyl-4-silyl entecavir;
9-[(1S,3R,4S)-4-Dimethylphenylsilyl-3-(benzyloxymethyl)-2-methylenecyclopentyl]guanine.
C27H31N5O2Si 485.65
USP Entecavir System Suitability Mixture RS
The mixture contains entecavir monohydrate and the following impurities (other impurities may also be present):
Entecavir 1-epimer.
8-Hydroxy entecavir.
8-Methoxy entecavir.
-
Entecavir Monohydrate CAS 209216-23-9 Assay 98....
-
Entecavir CAS 142217-69-4 API Factory High Qual...
-
Ganciclovir CAS 82410-32-0 API BW 759 GCV Antiv...
-
Acyclovir CAS 59277-89-3 Assay 98.0-101.0% (HPL...
-
Tiopronin CAS 1953-02-2 API Factory High Qualit...
-
Sorafenib Tosylate CAS 475207-59-1 Purity ≥99.0...
-
Eflornithine Hydrochloride Monohydrate CAS 9602...
-
Darunavir Ethanolate CAS 635728-49-3 Purity ≥99...
-
Darifenacin Hydrobromide CAS 133099-07-7 Assay ...
-
Dantrolene Sodium Salt Hydrate CAS 14663-23-1 A...
-
Daptomycin CAS 103060-53-3 Purity ≥95.0% API Fa...
-
Citicoline Sodium Salt Hydrate CAS 33818-15-4 A...
-
Cefotaxime Sodium Salt CAS 64485-93-4 Assay ≥91...
-
Cholestyramine CAS 11041-12-6 USP API Factory H...
-
Cinchonidine CAS 485-71-2 Assay 98.5%~101.0% AP...