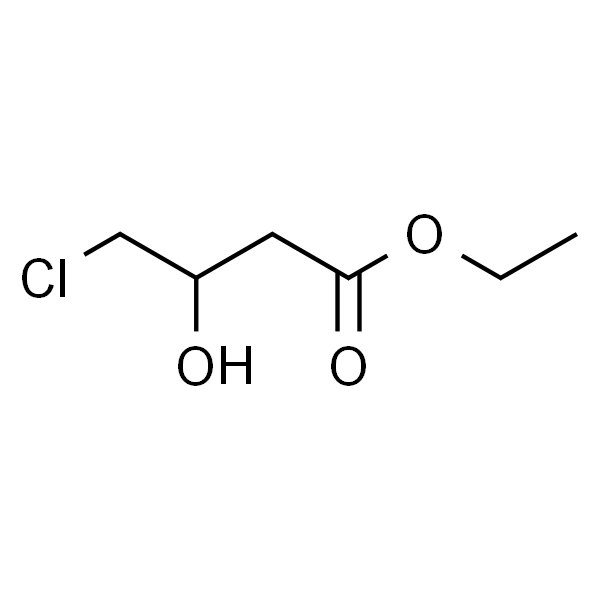
Ethyl 4-Chloro-3-Hydroxybutanoate CAS 10488-69-4 Assay ≥98.0% (GC) High Purity
Manufacturer Supply with High Purity and Stable Quality
Ethyl 4-Chloro-3-Hydroxybutanoate CAS 10488-69-4
Ethyl (S)-4-Chloro-3-Hydroxybutyrate CAS 86728-85-0
Ethyl (R)-(+)-4-Chloro-3-Hydroxybutyrate CAS 90866-33-4
Chiral Compounds, High Quality, Commercial Production
| Chemical Name | Ethyl 4-Chloro-3-Hydroxybutanoate |
| Synonyms | DL-Ethyl 4-Chloro-3-Hydroxybutyrate |
| CAS Number | 10488-69-4 |
| CAT Number | RF-CC187 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H11ClO3 |
| Molecular Weight | 166.6 |
| Melting Point | 136℃ |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless to Pale Yellow Liquid |
| Assay / Analysis Method | ≥98.0% (GC) |
| Ethyl 4-Chloroacetoacetate | ≤0.30% |
| Moisture (K.F) | ≤0.30% |
| Dichloromethane | ≤0.50% |
| Ethanol | ≤1.50% |
| pH | 5.0~7.0 |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediate |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Ethyl 4-Chloro-3-Hydroxybutanoate (CAS: 10488-69-4) with high quality, widely used in organic synthesis, synthesis of pharmaceutical intermediates and Active Pharmaceutical Ingredient (API) synthesis.
Ethyl 4-Chloro-3-Hydroxybutanoate (CAS: 10488-69-4) is a key chiral intermediate in statins. It is an intermediate of Olacetam, Olacetam in the treatment of alzheimer's disease. Ethyl 4-Chloro-3-Hydroxybutanoate (CAS: 10488-69-4), it is an important organic intermediate to synthesize precursor compounds of cholesterol-lowering drugs, but also for many activities synthesis of drugs such as hydroxymethylglutaryl reductase inhibitors and 1,4-dihydropyridine β-blockers. It has the advantage of being easy to synthesize and inexpensive, and it is a very cost-effective way to prepare Ethyll-4-Chloro-3-Hydroxy Butyrate by asymmetric reduction as a reaction raw material.
-
Ethyl 4-Chloro-3-Hydroxybutanoate CAS 10488-69-...
-
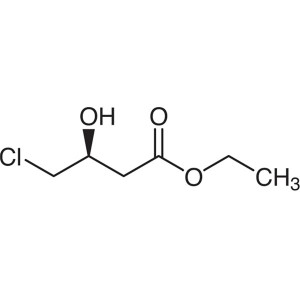
Ethyl (S)-4-Chloro-3-Hydroxybutyrate CAS 86728-...
-
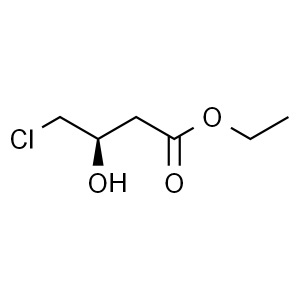
Ethyl (R)-(+)-4-Chloro-3-Hydroxybutyrate CAS 90...
-
Ethyl (R)-(-)-3-Hydroxybutyrate CAS 24915-95-5 ...
-
Ethyl (S)-(+)-3-Hydroxybutyrate CAS 56816-01-4 ...
-
Ethyl (S)-4-Chloro-3-hydroxybutyrate CAS 86728-...
-
Methyl (S)-(-)-2-Chloropropionate CAS 73246-45-...
-
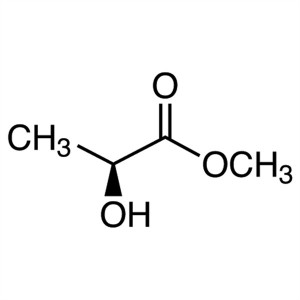
Methyl (S)-(-)-Lactate CAS 27871-49-4 Assay ≥99...
-
![Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate CAS 328086-60-8 PF-07321332 Boceprevir Intermediate](https://www.ruifuchem.com/uploads/CAS-328086-60-8-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)
Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrroli...
-
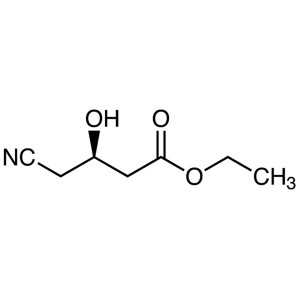
Ethyl (R)-(-)-4-Cyano-3-Hydroxybutyrate CAS 141...
-
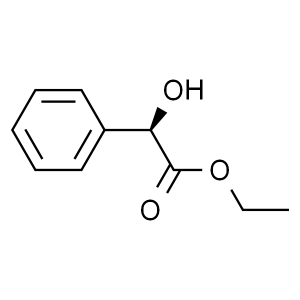
Ethyl (R)-(-)-Mandelate CAS 10606-72-1 Assay ≥9...
-
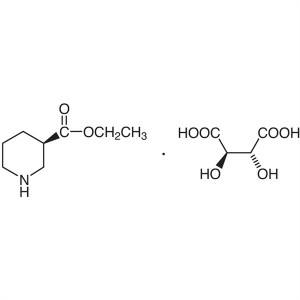
Ethyl (R)-Nipecotate L-Tartrate CAS 167392-57-6...
-
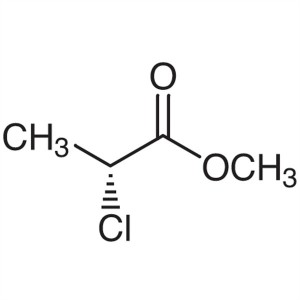
Methyl (R)-(+)-2-Chloropropionate CAS 77287-29-...
-
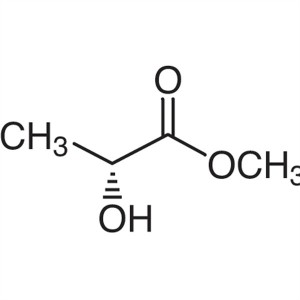
Methyl (R)-(+)-Lactate CAS 17392-83-5 Assay ≥99...
-
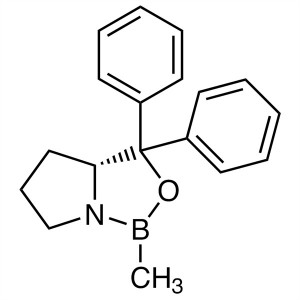
(R)-(+)-2-Methyl-CBS-oxazaborolidine; (R)-Me-CB...
-
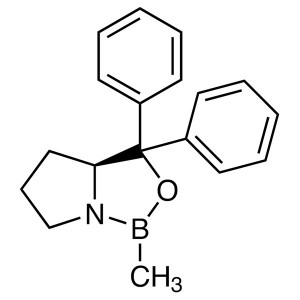
(S)-(-)-2-Methyl-CBS-Oxazaborolidine; (S)-Me-CB...