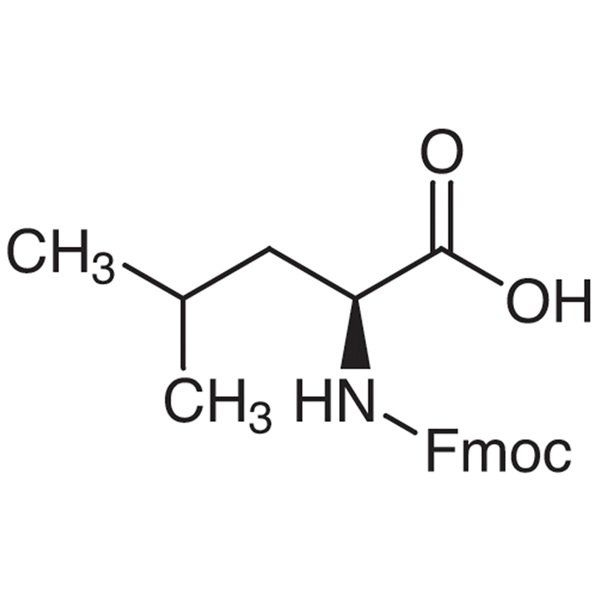
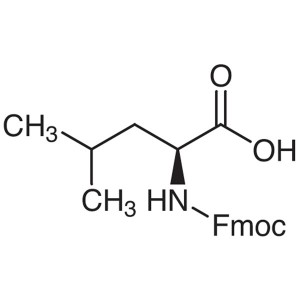
Fmoc-Leu-OH CAS 35661-60-0 N-Fmoc-L-Leucine Purity >99.0% (HPLC) Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of N-Fmoc-L-Leucine (Fmoc-Leu-OH) (CAS: 35661-60-0) with high quality. Ruifu Chemical supplys a series of amino acids. We can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase Fmoc-Leu-OH, Please e-mail: alvin@ruifuchem.com
| Chemical Name | N-Fmoc-L-Leucine |
| Synonyms | Fmoc-Leu-OH; Fmoc-L-Leu-OH; Fmoc-L-Leucine; N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-Leucine; N-(9-Fluorenylmethoxycarbonyl)-L-Leucine |
| Stock Status | In Stock, Production Capacity to Tons per Month |
| CAS Number | 35661-60-0 |
| Molecular Formula | C21H23NO4 |
| Molecular Weight | 353.42 g/mol |
| Melting Point | 153.0 to 160.0℃ |
| Density | 1.207±0.06 g/cm3 |
| Sensitive | Hygroscopic |
| Solubility in Methanol | Almost Transparency |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Category | Fmoc-Amino Acids |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White Powder | White Powder |
| Specific Rotation [α]20/D | -25.0°±2.0° (C=1 in DMF) |
-24.6° |
| Melting Point | 153.0 to 160.0℃ | 155.1℃ |
| TLC | ≥98.0% | >98.0% |
| Optical Purity | <0.30% D-Enantiomer | Complies |
| Clarity of Solution | 0.3 gram in 2ml DMF Clear Solution | Complies |
| Kaiser Test | <0.05% | <0.05% |
| Water by Karl Fischer | <0.50% | 0.12% |
| Loss on Drying | <0.50% (60℃, 2h) | 0.15% |
| Fmoc-β-Ala-OH | <0.20% (HPLC) | Complies |
| Fmoc-β-Ala-Leu-OH | <0.10% (HPLC) | Complies |
| Fmoc-Leu-Leu-OH | <0.10% (HPLC) | Complies |
| Fmoc-Ile-OH | <0.10% (HPLC) | Complies |
| Assay Free Amino Acid | <0.20% (GC) | Complies |
| Ethyl Acetate | <0.50% (GC) | Complies |
| Purity / Analysis Method | >99.0% (HPLC) | 99.85% |
| Mass Spectrum | In Accordance With the Standard | Complies |
| NMR Spectrum | In Accordance With the Standard | Complies |
| Conclusion | The product has been tested & complies with the specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry (2~8℃) warehouse away from incompatible substances. Protect from light and moisture.
Inspection procedure
1. Appearance
-- Visual inspection
2. Purity (HPLC)
2.1 Instrument
High performance liquid chromatography, PDA detector.
Electronic analytical balance
2.2 Reagent
Acetonitrile (chromatographic grade), Trifluoroacetic acid (chromatographic grade)
2.3 Chromatographic conditions
2.3.1 Column: YMC-ODS-AM, 5μL, 250x4.6 mm
2.3.2 Detection wavelength: INC220nm
Flow rate: 1.0mL/min
Sample size: 10μL (reference)
Diluent: acetonitrile
Data collection time:25.00min
2.4 Mobile phase preparation
Mobile phase A (0.1% trifluoroacetic acid water): accurately absorb 2.0ml trifluoroacetic acid, diluted with water to 2000ml, mix well, and degassing;
Mobile phase B (0.1% acetonitrile trifluoroacetic acid): accurately absorb 2.0ml trifluoroacetic acid, diluted to 2000ml with acetonitrile, mixing and degassing;
Time (min) A% B%
0.00 90 10
13.00 10 90
18.00 10 90
18.01 90 10
23.00 90 10
2.6 Preparation of sample solution
Weigh and dissolve 0.1g sample with acetonitrile and dilute to 100ml, shake well for use, or the same concentration. Prepare two samples in parallel.
2.7 Sample Determination
Analyze the sample according to the following sampling procedure:
More than 1 injection of blank solution
1 needle sample solution 1#
1 needle sample solution 2#
2.8 Result Calculation
2.8.1 The peak area normalization method was used to calculate the HPLC purity by deducting blank space.
2.8.2 The relative mean deviation of the purity of two needles shall not be greater than 1%
2.8.3 If the results of both injections meet the acceptance criteria, the average purity is taken as the final result.
3, Melting point -- RY-1 melting point instrument
4. Loss on drying test method
4.1 Instruments:
Electric thermostatic drying oven, 1/10,000 balance.
4.2 Procedure:
In a flat weighing bottle with a constant weight and over-drying ground mouth cover, weigh 1 gram (accurate to 0.0001 gram) of the sample. The sample should be evenly spread at the bottom of the weighing bottle with a thickness of no more than 10mm, put in a thermostatic electric drying oven, dry at 105~110℃ for 3 hours, and then move into the drying room to cool to room temperature for weighing.
Calculation: Loss on drying %= (M1-M2) ÷M×100
Where: M1: weight of sample and measuring bottle before drying, grams
M2: weight of sample and measuring bottle after drying, in grams
M: Sample weight, grams
5. Specific rotation
-- Specific rotation is measured in accordance with GB/T613-1988
Sample preparation: 0.5000g sample was accurately weighed and moved to a clean and dry 50ml volumetric bottle, 20ml DMF was added, the bottle was capped and shaken to dissolve, and then diluted to the scale with DMF.
Test: Adjust the zero of the gyroscope before the test, then load the test tube with the sample solution, record the rotation Angle, and calculate the specific rotation of the sample with the following formula.
[а]D20=(r×50) ÷ (L×W)
[а]D20: Specific optical rotation at 25℃ of sample solution
r: Optical rotation observed at 20℃ for the sample solution
50: Volume of prepared sample solution (ml)
w: Sample weight (g)
L: Optical rotation tube length (dm)
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description S22 - Do not breathe dust.
S24/25 - Avoid contact with skin and eyes.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
S27 - Take off immediately all contaminated clothing.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany 3
HS Code 2922491990
N-Fmoc-L-Leucine (Fmoc-Leu-OH) (CAS: 35661-60-0) is an amino acid derivative that can be prepared by the reaction of L-Leucine and 9-Fluorenomethoxycarbonyl Chloride.
Fmoc-amino acids, used in peptide synthesis, used as an organic synthesis intermediate, pharmaceutical intermediate, biochemical reagent or chemical reagent.
Fmoc-L-Leu-OH, is an amino acid derivative, used in peptide chemistry. Standard building block for routine solid phase peptide synthesis following the Fmoc-tactics. It is also one of the novel PPARγ ligands that can activate PPARγ in different ways, that reduces osteoclasts differentiation, and thus are better therapeutic targets in diabetes than traditional antidiabetic drugs.
Preparation 1.05g (0.008 mol) L-Leucine solid is dissolved in 10% sodium carbonate solution, stirred to fully dissolve glycine solid, and 9-fluorene methoxycarbonyl chloride (2.10g, 0.008 mol) solution dissolved in toluene (2~205 ml) is added dropwise at 20~30℃, drop addition for 30~60 minutes, end the dropwise addition, stir at 20~30℃ for 1~8 hours, add 30-200 ml of water to dilute, and extract with n-butyl acetate (80 ml) to remove excess 9-fluorene methoxycarbonyl chloride, The obtained aqueous phase is acidified with concentrated hydrochloric acid to PH = 0.5~3.5, and then extracted with n-butyl acetate (80 ml), and the obtained oil phase is washed with water to remove hydrochloric acid, the oil phase was concentrated to remove n-butyl acetate solvent, white crystals were precipitated, filtered and dried to obtain 2.46g of Nα-9-fluorene methoxycarbonyl-L-leucine, with an yield of 87.0%.