Good User Reputation for Irinotecan - Lurasidone Hydrochloride CAS 367514-88-3 API High Purity – Ruifu
Good User Reputation for Irinotecan - Lurasidone Hydrochloride CAS 367514-88-3 API High Purity – Ruifu Detail:
Manufacturer Supply with High Purity and Stable Quality
Lurasidone Hydrochloride (CAS 367514-88-3) Related Intermediates:
(1R,2R)-1,2-Cyclohexanedimethanol CAS 65376-05-8
(3aR,4S,7R,7aS)-rel-Hexahydro-4,7-methano-1H-isoindole-1,3(2H)-dione CAS 14805-29-9
Lurasidone Hydrochloride CAS 367514-88-3 API
High Quality, Commercial Production
| Chemical Name | Lurasidone Hydrochloride |
| Synonyms | Lurasidone HCl; Latuda |
| CAS Number | 367514-88-3 |
| CAT Number | RF-API64 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C28H36N4O2S |
| Molecular Weight | 492.684 |
| Melting Point | 198.0~205.0°C |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Crystalline Powder |
| NMR | Consistent with Structure |
| Solubility | Meets the Requirements |
| Max. Single Impurity | ≤0.20% |
| Total Impurities | ≤0.50% |
| Moisture (K.F) | ≤1.0% |
| Residue of Ignition | ≤0.20% |
| Heavy Metals | ≤20ppm |
| Purity | ≥99.0% |
| Test Standard | Enterprise Standard |
| Usage | API, Atypical Antipsychotic Drug (AAPD) |
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Lurasidone Hydrochloride (CAS 367514-88-3) with high quality, API.
Lurasidone Hydrochloride (CAS 367514-88-3) is an orally administered substance that belongs to the class of atypical antipsychotic drugs (AAPD), developed by Dainippon Sumitomo Pharma. It was approved by the U.S. Food and Drug Administration (FDA) for treatment of schizophrenia on October 29, 2010 and is currently pending approval for the treatment of bipolar disorder in the United States. It received approval by the EMA in 2014 for schizophrenia. Lurasidone has also been launched in a number of countries outside the US and EU, including Canada, where it is approved for both indications. It is produced through a multi-step chemical process.
Product detail pictures:
Related Product Guide:
Which has a positive and progressive attitude to customer's desire, our corporation constantly improves our merchandise quality to satisfy the desires of consumers and further focuses on safety, reliability, environmental demands, and innovation of Good User Reputation for Irinotecan - Lurasidone Hydrochloride CAS 367514-88-3 API High Purity – Ruifu , The product will supply to all over the world, such as: Georgia, Bogota, Mongolia, Our items are exported worldwide. Our customers are always satisfied with our reliable quality, customer-oriented services and competitive prices. Our mission is "to continue to earn your loyalty by dedicating our efforts to the constant improvement of our merchandise and services in order to ensure the satisfaction of our end-users, customers, employees, suppliers and the worldwide communities in which we cooperate".
-
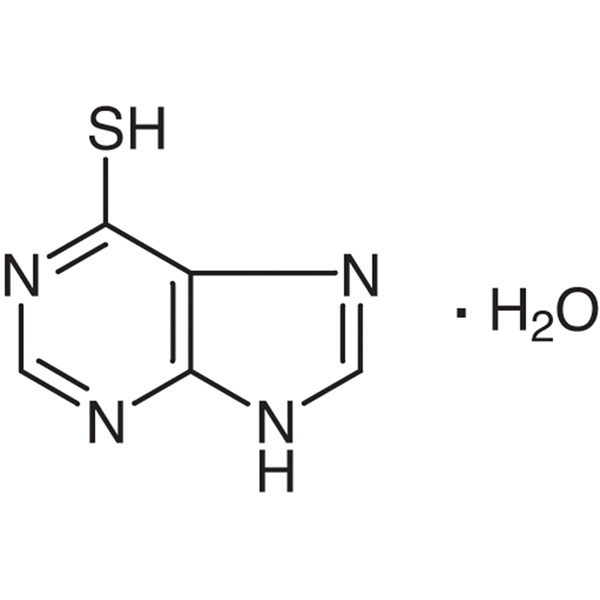
Good Quality Pharmaceutical Intermediates - 6-...
-
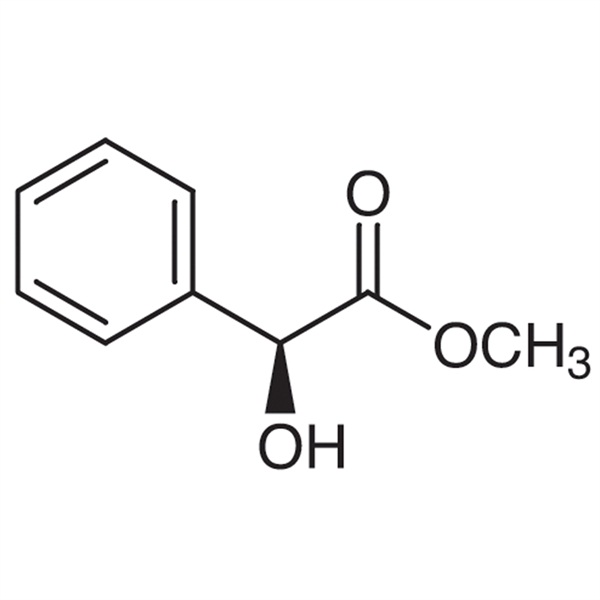
PriceList for R-Glycidyl Tosylate - (S)-(+)-Me...
-
Rapid Delivery for Cyclocytidine HCl - 2-Cyano...
-
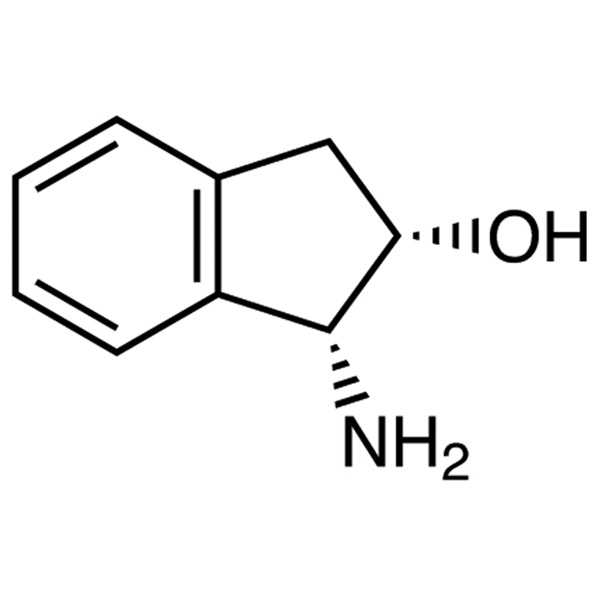
Wholesale Dealers of (R)-(-)-α-Chlorohydrin - ...
-
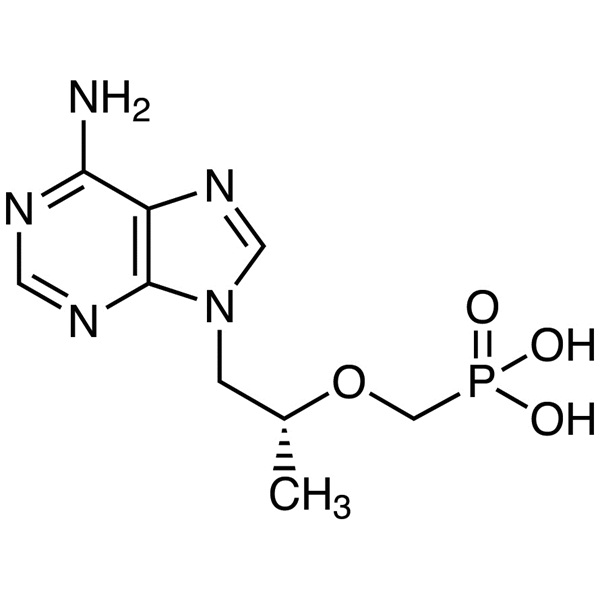
2021 wholesale price Tenofovir - Tenofovir CAS...
-
Factory making 4-Chloro-N-Methyl-2-Pyridinecarb...
In China, we have many partners, this company is the most satisfying to us, reliable quality and good credit, it is worth appreciation.
