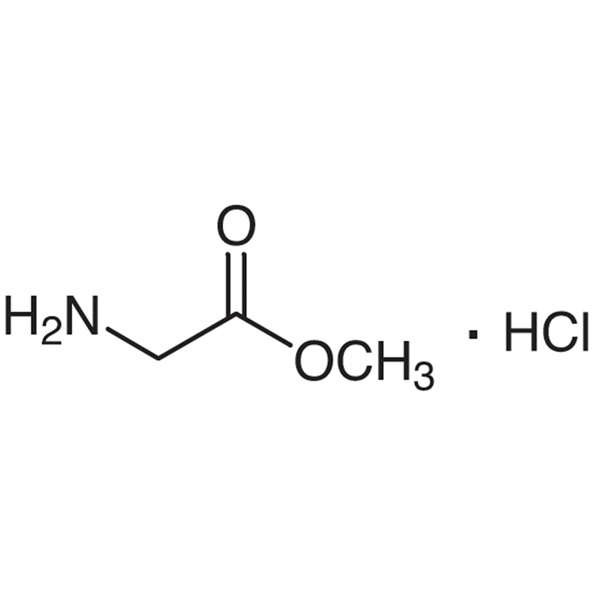
H-Gly-OMe·HCl CAS 5680-79-5 Glycine Methyl Ester Hydrochloride Assay >99.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Glycine Methyl Ester Hydrochloride (H-Gly-OMe·HCl) (CAS: 5680-79-5) with high quality. Ruifu Chemical supplys a series of amino acids and derivatives. We can provide worldwide delivery, small and bulk quantities available. Purchase H-Gly-OMe·HCl, Please contact: alvin@ruifuchem.com
| Chemical Name | Glycine Methyl Ester Hydrochloride |
| Synonyms | H-Gly-OMe·HCl; Gly-OMe·HCl; Methyl Glycinate Hydrochloride; Methyl Glycinate HCl; Aminoacetic Acid Methyl Ester Hydrochloride; Glycinate Methyl Ester Hydrochloride |
| Stock Status | In Stock, Production Capacity 30 Tons per Month |
| CAS Number | 5680-79-5 |
| Molecular Formula | C3H7NO2·HCl |
| Molecular Weight | 125.55 g/mol |
| Melting Point | 170.0~178.0℃ |
| Density | 1.000 |
| Sensitive | Moisture Sensitive |
| Solubility in Water | Soluble in Water, Almost Transparency |
| Solubility | Soluble in Alcohol |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Category | Amino Acids and Derivatives |
| Brand | Ruifu Chemical |
| Safety Statements | S22 - Do not breathe dust. S24/25 - Avoid contact with skin and eyes. | ||
| WGK Germany | 3 | HS Code | 2922491990 |
| Items | Inspection Standards | Results |
| Appearance | White Crystalline Powder | Complies |
| Melting Point | 170.0~178.0℃ | 174.4℃ |
| Loss on Drying | ≤0.50% | 0.37% |
| Burning Residue | ≤0.10% | <0.10% |
| Sulfate (SO42-) | ≤0.020% | 0.013% |
| Glycine Hydrochloride | ≤1.00% | 0.82% |
| Heavy Metals (as Fe) | ≤10ppm | <10ppm |
| Free Hydrochloric Acid | ≤0.20% | 0.162% |
| H-Gly-OMe·HCl Assay | >99.0% (Titration by AgNO3) | 99.2% |
| Mass Spectrum | In Accordance With the Standard | Complies |
| NMR Spectrum | In Accordance With the Standard | Complies |
| PH Value (15% Water/25℃) | 1.0~2.0 | Complies |
| Conclusion | The product has been tested & complies with the specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry (2~8℃) warehouse away from incompatible substances. Protect from light and moisture.
1. Properties: This product should be white crystalline powder.
2. Assay:
1) Determination of Glycine Methyl Ester Hydrochloride assay
1.1 Method
Under acidic conditions, the sample reacts with a certain amount of silver nitrate, and the excess silver nitrate is revertitrated with ammonium thiocyanate, referring to ammonium ferric sulfate
As indicator, the total chlorine content was determined according to the volume of the standard solution consumed, and the total chlorine content was subtracted from the content of glycine hydrochloride to obtain Glycine Methyl Ester Hydrochloride content.
1.2 Reagents
Dibutyl phthalate;
Nitric acid solution: 2+3;
Silver nitrate standard titration solution: c(AgNO3) = 0.1 mol/L
Ammonium thiocyanate standard titration solution: c(NH4SCN) = 0.1 mol/L
Ammonium ferric sulfate (III) indicator liquid: 80 g/L
1.3 Analysis Procedure
Weigh about 0.5g sample, accurate to 0.0001g. Place in a 250ml conical flask, dissolve in 40ml water, add 10 ml nitric acid solution, Add (50 ± 0.02) mL silver nitrate standard titration solution, 3mL ammonium ferric sulfate indicator solution, 4mL dibutyl phthalate, and thiocyanate Ammonium standard titration solution was titrated until the solution was light brown and remained colourless for 30 s.
1.4 Result calculation
The mass fraction of glycine methyl ester hydrochloric acid content w1, which is expressed as %, is calculated according to Equation (1) :
w1= (c1V1-c2V2)*M/1000/m * 100-w2 *1.126 formula (1)
Where:
V1: Volume of silver nitrate standard titration solution (ml)
V2: Volume of ammonium thiocyanate standard titration solution consumed by titration (ml)
c1: Exact value of silver nitrate standard titration solution (mol/L)
c2: Exact value of ammonium thiocyanate standard titration solution (mol/L)
w2: Mass fraction (%) of glycine hydrochloride content as determined in Section 4.2
M: Molar mass of glycine methyl ester hydrochloride (g/mol)
1.126: Conversion coefficient of glycine hydrochloride to Glycine methyl ester hydrochloride.
The arithmetic mean value of two parallel measurement results was taken as the measurement result, and the absolute difference between the two parallel measurement results was no more than 0.05%.
2) Determination of glycine hydrochloride content
2.1 Method
Using dimethyl yellow as an indicator, the sample was titrated with sodium hydroxide standard titrating solution, and the volume of the solution was titrated according to the consumption of sodium hydroxide standard.
Calculate the glycine hydrochloride content.
2.2 Reagent
Standard titration solution of sodium hydroxide: c (NaOH) = 0.1mol/L
Dimethyl yellow indication liquid: 10 g/L
2.3 Analysis Procedure
Weigh about 5.0 g laboratory sample, accurate to 0.0001g, into a 200 ml beaker, dissolve with 50ml water, and add 5 drops of dimethyl
Yellow indication liquid, with sodium hydroxide standard titration solution titration until the solution from red to orange yellow is the end point
2.4 Result Calculation
The mass fraction w2 of glycine hydrochloride content is expressed as % and calculated according to Equation (2):
w2 =(V/1000)cM/m *100 formula (2)
Where:
V: Volume of sodium hydroxide standard titration solution (mL)
c: Exact value of sodium hydroxide standard titration solution concentration (mol/L)
M: Molar mass of glycine hydrochloride (g/mol)
m: Mass value of the sample (g)
The arithmetic mean value of two parallel measurement results was taken as the measurement result, and the absolute difference between the two parallel measurement results was no more than 0.05%
3. Melting point: According to the method of melting point determination (Chinese Pharmacopoeia 2010 Appendix VIC), the melting point range is 172-178 ºC.
4. Drying weight loss: take this product at 105.0℃ and dry it to constant weight. The weight loss shall not exceed 0.5%.
5. Storage: This product should be refrigerated away from light.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Glycine Methyl Ester Hydrochloride (H-Gly-OMe·HCl) (CAS: 5680-79-5) is useful as a raw material for household insecticides, pyrethroids, and raw materials for the pharmaceutical industry. glycine methyl ester hydrochloride is an important intermediate in the preparation of chrysanthemum acid and dichloro-Chrysanthemum acid, and is also an intermediate of fungicide isourea. used as household insecticide, pyrethroid raw materials and pharmaceutical industry raw materials used in medicine, food, organic synthesis, etc.
Production method may be prepared using glycine or chloroacetic acid as a starting material. As described below. Glycine as raw material method with dry hydrogen chloride gas into anhydrous ethanol, first made into hydrogen chloride ethanol solution, and then add glycine, heating and stirring for esterification, the reaction temperature is controlled at 80~85℃, after glycine was completely dissolved, the mixture was stirred for several minutes, and the reaction solution was transferred to a crystallization pan while hot, cooled to crystallize, and filtered to obtain glycine ethyl ester hydrochloride. Reaction equation: NH2CH2COOH C2H5O[HCl]→ NH2CH2COOC2H5·HCl can also be added with glycine and anhydrous ethanol in the reactor with stirring, heated to 60℃, hydrogen chloride gas, hydrogen chloride is passed to the end point when the reactant is completely dissolved and hydrogen chloride is continued to recrystallize. The reaction solution is transferred to the crystallization pan, and the crystallization is cooled while stirring, filtered, washed with absolute ethanol, the glycine ethyl ester hydrochloride was obtained by drying. Chloroacetic acid as raw material to chloroacetic acid, urotropine and anhydrous ethanol was added to the reactor, at 45℃ when human ammonia, pH = 8~8.5, control the rate of ammonia, in order to maintain the reaction temperature between 40 to 65℃, after the ammonia gas was passed, the reaction was maintained at 65℃. For 1H, and then the temperature was lowered to 30℃, and the hydrogen chloride-anhydrous ethanol solution was added dropwise. After the dropwise addition, react at 70℃ for 2H, and then filter at 62~65℃ while hot to separate ammonium chloride crystals. The filtrate is placed in a crystallization Pan, cooled to 10℃, and stirred, edge crystallization, Crystal filtration, drying to glycine ethyl ester hydrochloride. Reaction equation: ClCH2COOH NH3 [ethanol] → NH2CH2COOH[C2H5OH]→[HCl]NH2CH2COOH·HCl, methyl glycinate hydrochloride can be obtained by replacing ethanol with methanol. Reaction equation: NH2CH2COOH CH3OH[HCl]→NH2CH2COOCH3·HCl
-
D-Prolinol CAS 68832-13-3 Purity >99.0% (GC) E....
-
H-D-Val-OtBu·HCl CAS 104944-18-5 D-Valine tert-...
-
L-2-Aminobutyric Acid CAS 1492-24-6 (H-Abu-OH) ...
-
Fmoc-D-Phe(4-NH2)-OH CAS 324017-21-2 Fmoc-4-Ami...
-
Boc-D-Ala-NH2 CAS 78981-25-6 Assay ≥98.0% (HPLC)
-
L-Tyrosinamide CAS 4985-46-0 (H-Tyr-NH2) Purity...