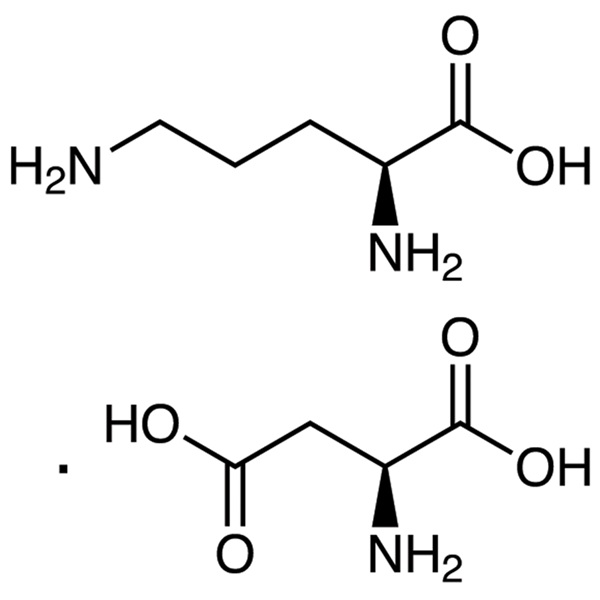
L-Ornithine L-Aspartate CAS 3230-94-2 (L-Orn-L-Asp) Assay 98.0~102.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of L-Ornithine L-Aspartate (L-Orn-L-Asp) (CAS: 3230-94-2) with high quality, production capacity 5000 Tons per year. Ruifu Chemical supplys a series of amino acids & derivatives. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in L-Ornithine L-Aspartate, Please contact: alvin@ruifuchem.com
| Chemical Name | L-Ornithine L-Aspartate |
| Synonyms | L-Orn-L-Asp; L-Ornithine L-Aspartate Salt; Ornithine Aspartate; L-Ornithine Aspartate; Laevo-Ornithine Laevo-Aspartate; L-Aspartic Acid, compd. with L-Ornithine (1:1); Ornithine L-Form Aspartate; Aspartic Acid Compound with Ornithine; (S)-2,5-Diaminopentanoic Acid L-Aspartate Salt; LOLA |
| Stock Status | In Stock |
| CAS Number | 3230-94-2 |
| Molecular Formula | C5H12N2O2·C4H7NO4 |
| Molecular Weight | 265.27 |
| Melting Point | 202.0 to 206.0℃ |
| Sensitive | Hygroscopicity. Heat Sensitive |
| Water Solubility | Very Soluble in Water or Acetic Acid, Very Slightly Soluble in Methanol or Ethanol, and Almost Insoluble in Chloroform or Acetone |
| Storage Temp. | Sealed in Dry, Store at Room Temperature |
| COA & MSDS | Available |
| Classification | Amino Acids & Derivatives |
| Brand | Ruifu Chemical |
| Hazard Codes | Xi - Irritant | WGK Germany | 2 |
| Risk Statements | 36/37/38 | RTECS | CI9463000 |
| Safety Statements | 26-37/39-24/25 | HS Code | 2922491990 |
| Items | Inspection Standards | Results |
| Appearance | White Crystals or Crystalline Powder; Odorless and Hygroscopic | Conforms |
| Identification | Infrared Absorption Spectrum | Conforms |
| Specific Rotation [α]20/D | +27.0° to +30.0°(C=8, 6mol/L HCl) |
+27.7° |
| State of Solution (Transmittance) | Clear and Colorless ≥98.0% | 98.3% |
| Chloride (Cl) | ≤0.030% | <0.030% |
| Sulfate (SO4) | ≤0.020% | <0.020% |
| Ammonium (NH4) | ≤0.020% | <0.020% |
| Iron (Fe) | ≤30ppm | <30ppm |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Arsenic (As2O3) | ≤2.0ppm | <2.0ppm |
| Other Amino Acids | Conforms | Conforms |
| Water (by Karl Fischer) | ≤7.00% | 2.60% |
| Residue on Ignition (Sulfated) | ≤0.20% | 0.04% |
| Assay | 98.0 to 102.0% (Titration: Anhydrous Basis) | 99.7% |
| pH Test | 5.5 to 7.0 | 5.7 |
| Conclusion | This Product by Inspection Accords with the Standard of AJI97 | |
| Main Uses | Pharmaceuticals; Liver Diseases | |
Package: Fluorinated Bottle, 25kg/bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool, dry and ventilated warehouse away from incompatible substances. Protect from light and moisture.
3230-94-2 - Standard
Calculated as dried product, containing no less than 98.0% of C5H12N2O2·C4H7NO4.
3230-94-2 - Trait
This product is white crystals or crystalline powder; Odorless, with hygroscopicity.
This product is very soluble in water or acetic acid, very slightly soluble in methanol or ethanol, and almost insoluble in chloroform or acetone.
Specific rotation
Take this product, precision weighing, add hydrochloric acid solution (6-10) to dissolve and dilute the solution containing 80mg per lm l, determination according to law (General 0621), the specific rotation was 27.0° to 30.0°.
3230-94-2 - Differential diagnosis
Take about 10 mg of this product, add 2ml of water to dissolve, add about 2mg of ninhydrin, heat, the solution shows blue purple.
The infrared absorption spectrum of this product should be consistent with that of the reference product (General rule 0402).
3230-94-2 - Exam
Acidity
Take 0.5g of this product, add water 20ml to dissolve, according to the law (General 0631), pH value should be 6.0~7.0.
Transmittance of solution
Take 0.5g of this product, add water 20ml to dissolve, according to UV-visible spectrophotometry (General rule 0401), determine the transmittance at 430mn wavelength, not less than 98.0%.
chloride
Take 0.10g of this product and check it according to law (General rule 0801). Compared with the control solution made of 0.03% of standard sodium chloride solution, it should not be more concentrated ().
Sulfate
Take 1.0g of this product and check it according to law (General rule 0802). Compared with the control solution made of 0.02% of standard potassium sulfate solution, it should not be more concentrated ().
Ammonium salt
Take 0.10g of this product and check it according to law (General rule 0808). Compared with the control solution made of 0.04% of standard ammonium chloride solution, it shall not be deeper ().
Loss on drying
Take this product, dry to constant weight at 120°C, weight loss shall not exceed 7.0% (General rule 0831).
Ignition residue
This product 1.0 g, inspection according to law (General 0841), residue shall not exceed 0.2%.
Iron Salt
Take 0.5g of this product and check it according to law (General rule 0807). Compared with the control solution made of 0.003% of standard iron solution, it should not be deeper ().
Heavy metals
Take this product 1.0g, inspection according to law (General Principles 0821 second law), including heavy gold shall not exceed 10 parts per million.
Arsenic salt
Take 1.0g of this product, add 23ml of water to dissolve, add 5ml of hydrochloric acid, check according to law (General rule 0822 first law), should comply with the provisions (0.0002%).
3230-94-2 - Content determination
Take this product about 70mg, precision weighing, add anhydrous formic acid 5ml and ice fermented acid 50ml dissolved, according to the potential titration method (General rule 0701), with perchloric acid titration solution (0.1 mol/L) titration, and the results of the titration were corrected with a blank test. Each 1 ml of perchloric acid titration solution (0.1 mol/L) corresponds to 8 84mg of C5H12N202·C4H7NO4.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
1. L-Ornithine L-Aspartate (L-Orn-L-Asp) (CAS: 3230-94-2) was developed in Germany in the 1960s and was the first clinically used for the treatment of alcoholism and hepatic encephalopathy. With the accumulation of clinical application experience, Ornithine Aspartate has been more widely used in the treatment of liver diseases, and has achieved definite curative effects on hepatic encephalopathy, drug-induced liver damage, fatty liver, chronic hepatitis and other diseases. , Has been widely recognized by clinicians. Ornithine aspartate is a stable dipeptide compound prepared by chemical synthesis of L-ornithine and L-aspartic acid, which is extremely soluble in water and almost insoluble in methanol or ethanol. When this product enters the human body, it is decomposed into aspartic acid and ornithine, which can directly participate in liver cell metabolism, and can activate two key enzymes in the liver's detoxification function, thereby helping to remove harmful free radicals and enhance The liver's detoxification function quickly reduces excessive blood ammonia and promotes the repair and regeneration of liver cells, thereby effectively improving liver function and restoring the body's energy balance.
2. L-Ornithine L-Aspartate promotes the speed of cell metabolism in the whole body, accelerates blood circulation, can eliminate toxins and wastes deposited in the body, can promote the metabolism of liver cells, and can also meet the needs of liver cell growth and development energy.
3. L-Ornithine L-Aspartate strengthens the function of liver cells by accelerating the cycle of ornithine, can quickly reduce excessive blood ammonia within a few hours, correct the decompensation of amino acids, and improve the brain Symptoms.
4. L-Ornithine L-Aspartate can increase the energy synthesis of hepatocytes,Aspartate can participate in the synthesis of nucleic acid in hepatocytes, which is beneficial to repair damaged hepatocytes.
5, Most amino acids are used as building blocks to make protein. But L-ornithine-L-aspartate is not used to make protein. Instead it is broken down in the body to provide ornithine and aspartic acid.
6. Recovery in the treatment of liver cirrhosis, diseases, and enhance physical fitness.Pharmaceutical intermediates, biochemical research, compound amino acid combination formula, raw materials for peptide synthesis.Culture, enhance human immunity, resistance to fatigue, beneficial to human body health.
-
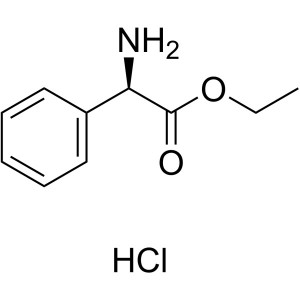
H-D-Phg-OEt·HCl CAS 17609-48-2 Assay ≥98.0% (HPLC)
-
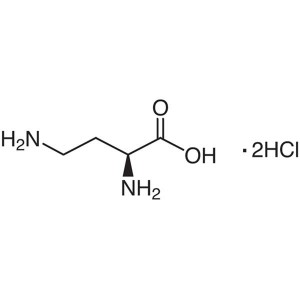
L-2,4-Diaminobutyric Acid Dihydrochloride CAS 1...
-
L-(-)-Methioninol CAS 2899-37-8 (H-Met-Ol) Puri...
-
Boc-Arg(Z)2-OH CAS 51219-19-3 Purity >98.0% (HPLC)
-
1-Boc-Piperazine CAS 57260-71-6 Purity >99.5% (...
-
Fmoc-Gly-OH CAS 29022-11-5 Fmoc-Glycine Purity ...