Lithium Aluminum Hydride (LiAlH4) CAS 16853-85-3 Purity >97.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Lithium Aluminum Hydride (LAH; LiAlH4) (CAS: 16853-85-3) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Lithium Aluminum Hydride, Please contact: alvin@ruifuchem.com
| Chemical Name | Lithium Aluminum Hydride |
| Synonyms | LAH; LiAlH4; Aluminum Lithium Hydride |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 16853-85-3 |
| Molecular Formula | LiAlH4 |
| Molecular Weight | 37.95 g/mol |
| Melting Point | 125℃(dec.)(lit.) |
| Density | 0.97 g/mL at 20℃ |
| Store Under Inert Gas | Store Under Inert Gas |
| Sensitive | Air & Moisture Sensitive |
| Solubility | Soluble in Dioxane, Ether, Tetrahydrofuran |
| Hydrolytic Sensitivity | 8: Reacts Rapidly with Moisture, Water, Protic Solvents |
| Stability | Stable. Reacts violently with water, liberating hydrogen. Incompatible with strong oxidizing agents, alcohols, acids. |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Powder | White Powder |
| Purity (Active Hydrogen) | >97.0% | 97.43% |
| X-Ray Diffraction | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: 500g/tin, 1kg/tin, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire; avoid moisture. The solid and solution of Lithium Aluminum Hydride are highly flammable and must be isolated from air and moisture, and it is best stored in a nitrogen atmosphere.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes R15 - Contact with water liberates extremely flammable gases
R34 - Causes burns
R14/15 -
R11 - Highly Flammable
R36/37 - Irritating to eyes and respiratory system.
R19 - May form explosive peroxides
R40 - Limited evidence of a carcinogenic effect
R10 - Flammable
R67 - Vapors may cause drowsiness and dizziness
R66 - Repeated exposure may cause skin dryness or cracking
R22 - Harmful if swallowed
R12 - Extremely Flammable
R35 - Causes severe burns
R37 - Irritating to the respiratory system
R65 - Harmful: May cause lung damage if swallowed
R48/20 -
R63 - Possible risk of harm to the unborn child
R36/38 - Irritating to eyes and skin.
R61 - May cause harm to the unborn child
R60 - May impair fertility
Safety Description S43 - In case of fire use ... (there follows the type of fire-fighting equipment to be used.)
S7/8 -
S6A -
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S43B -
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
S33 - Take precautionary measures against static discharges.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S16 - Keep away from sources of ignition.
S24/25 - Avoid contact with skin and eyes.
S27 - Take off immediately all contaminated clothing.
S29 - Do not empty into drains.
S62 - If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label.
S53 - Avoid exposure - obtain special instructions before use.
UN IDs UN 3399 4.3/PG 1
WGK Germany 2
RTECS BD0100000
FLUKA BRAND F CODES 10-21
TSCA Yes
Hazard Class 4.3
Packing Group I
Lithium Aluminum Hydride (LAH; LiAlH4) (CAS: 16853-85-3) is a promising compound for hydrogen storage, with a high gravimetric and volumetric hydrogen density and a low decomposition temperature. Similar to other metastable hydrides, LiAlH4 does not form by direct hydrogenation at reasonable hydrogen pressures; therefore, there is considerable interest in developing new routes to regenerate the material from the dehydrogenated products LiH and Al.
Lithium Aluminum Hydride is a commonly used reducing reagent in organic chemistry, which can reduce a variety of functional group compounds; it can also act on double bond and triple bond compounds to achieve hydride aluminum reaction;
Lithium Aluminum Hydride can also be used as a base to participate in the reaction. Lithium Aluminum Hydride has a strong hydrogen transfer ability, which can reduce aldehydes, esters, lactones, carboxylic acids, and epoxides to alcohols, or convert amides, imine ions, nitriles and aliphatic nitro compounds into corresponding amines .
In addition, the super reduction ability of Lithium Aluminum Hydride makes it possible to act on other functional groups, such as reducing halogenated alkanes to alkanes. In this type of reaction, the activity of halogenated compounds is iodine, bro-mine and chlorinated in descending order.
Lithium Aluminum Hydride is used as a powerful reducing agent inorganic synthesis. Except for olefinic doublebonds, almost all organic functional groupsare reduced by Lithium Aluminum Hydride (Sullivan and Wade 1980). It is used extensivelyin pharmaceutical synthesis and in catalytichydrogenation.
Used to make other chemicals, as a polymerization catalyst, as a hydrogen source, and as a propellant.
Used as a reducing agent in pharmaceutical, perfume, pesticide, dye and other fine organic synthesis.
Lithium Aluminum Hydride (LAH; LiAlH4) (CAS: 16853-85-3) is a highly flammable solid and may ignite in moist or heated air. Exposure to water results in the release of hydrogen, which can be ignited by the heat from the exothermic reaction. Lithium aluminum hydride should not be used as a drying agent for solvents because fires can easily result (LAH decomposes at about 125° C, a temperature easily reached at a flask's surface in a heating mantle). The decomposition products of LAH can be quite explosive, and the products of its reaction with carbon dioxide have been reported to be explosive. Use dry chemical powder or sand to extinguish fires involving lithium aluminum hydride. Never use water or carbon dioxide extinguishers on an LAH fire.
Lithium Aluminum Hydride (LAH; LiAlH4) (CAS: 16853-85-3) should be handled in areas free of ignition sources under an inert atmosphere. Safety glasses, impermeable gloves, and a fire-retardant laboratory coat are required. A dry powder fire extinguisher or pail of sand (and shovel) must be available in areas where LAH is to be handled or stored. Work with large quantities of powdered LAH should be conducted in a fume hood under an inert gas such as nitrogen or argon. Lithium aluminum hydride should be stored in tightly sealed containers in a cool, dry area separate from combustible materials. Dry LAH powder should never be exposed to water or moist air. Lithium aluminum hydride can be a finely powdered reagent that produces a reactive dust on handling. The older practice of grinding lithium aluminum hydride prior to use can cause explosions and should not be employed.
UN1410 Lithium Aluminum Hydride (dry), Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. UN1411 Lithium Aluminum Hydride, ethereal, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material, 3- Flammable liquid.
-

Lithium Aluminum Hydride (LiAlH4) CAS 16853-85-...
-
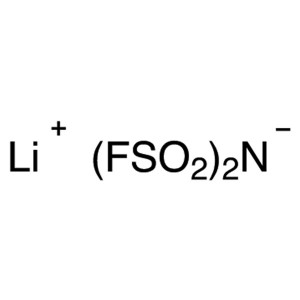
Lithium Bis(fluorosulfonyl)imide (LiFSI) CAS 17...
-
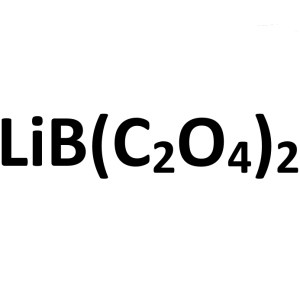
Lithium Bis(oxalate)borate (LiBOB) CAS 244761-2...
-
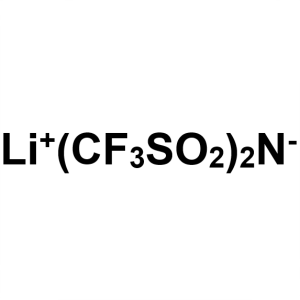
Lithium Bis(trifluoromethanesulphonyl)imide (Li...
-
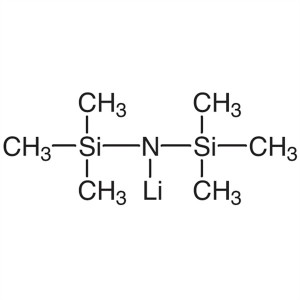
Lithium Bis(trimethylsilyl)amide (LiHMDS) CAS 4...
-

Lithium Bromide CAS 7550-35-8 Purity >99.0% (Ti...
-
Lithium Carbonate (Li2CO3) CAS 554-13-2 Purity ...
-

Lithium Chloride Anhydrous CAS 7447-41-8 Purity...
-
Lithium Difluoro(oxalato)borate (LiDFOB) CAS 40...
-
Lithium Difluorophosphate (LiPO2F2 / LiDFP) CAS...
-

Lithium Hexafluorophosphate (LiPF6) CAS 21324-4...
-

Lithium Hydride (LiH) CAS 7580-67-8 Purity >98.0%
-

Lithium Hydroxide Anhydrous (LiOH) CAS 1310-65-...
-
Lithium Hydroxide Monohydrate CAS 1310-66-3 LiO...
-

Lithium Tetrafluoroborate (LiBF4) CAS 14283-07-...


