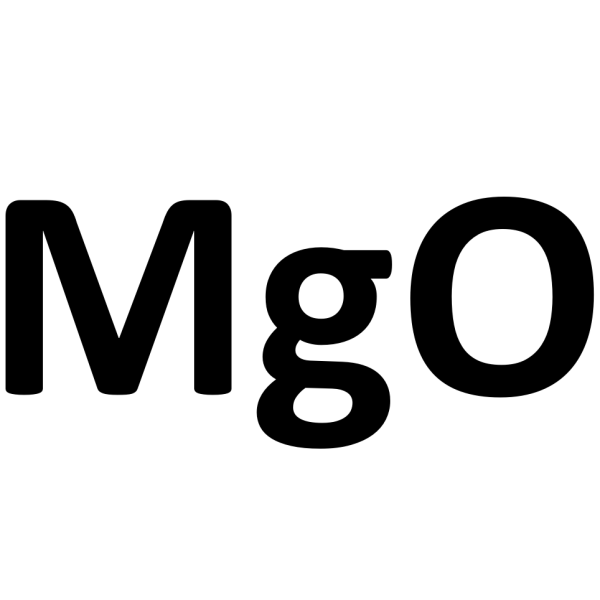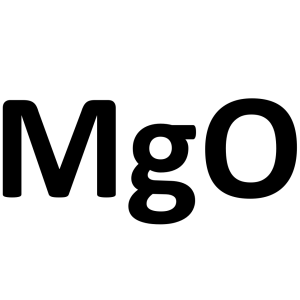Magnesium Oxide MgO CAS 1309-48-4 Pharmaceutical Grade 96.0%-100.5% (After Ignition)
Shanghai Ruifu Chemical Co., Ltd. is the leading supplier of Magnesium Oxide (MgO) (CAS: 1309-48-4) with high quality, production capacity 10000 tons per year. Ruifu Chemical has been supplying Magnesium Oxide more than 15 years. Magnesium Oxide is available in different grades for different applications, such as light grade, high purity light grade, high purity heavy grade, active grade, screen glass grade, rubber grade, conductive plastic grade, catalyst grade, silicon-steel grade, pharmaceutical grade; GFRP (glass fiber reinforced plastic) grade, lubricating oil grade, fluorescence grade, electrical grade.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Magnesium Oxide (MgO), please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Magnesium Oxide |
| Synonyms | MgO |
| Pharmaceutical Grade | Meet the Requirements of USP / Chinese Pharmacopoeia Standards |
| Stock Status | In Stock, Total Production Capacity 10000 Tons per Year |
| CAS Number | 1309-48-4 |
| Molecular Formula | MgO |
| Molecular Weight | 40.30 g/mol |
| Melting Point | 2852℃ |
| Boiling Point | 3600℃/1000 hPa |
| Density | 3.58 g/cm3 at 25℃ |
| Note | Hygroscopic and a Noncombustible Substance |
| Storage Temperature | Store Long-Term in a Cool and Dry Place |
| COA & MSDS | Available |
| Sample | Available |
| Origin of Product | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications |
| Appearance | White Powder |
| Magnesium Oxide (MgO) | 96.0%-100.5% (After Ignition) |
| Calcium Oxide (CaO) | ≤1.10% |
| Loss on Ignition | ≤5.00% |
| Free Alkali and Soluble Salts | ≤2.00% |
| Acid-Insoluble Substances | ≤0.10% |
| Iron | ≤0.05% |
| Manganese Content | ≤0.003% |
| Chloride | ≤0.14% |
| Sulphates | ≤0.30% |
| Heavy Metals (as Pb) | ≤20ppm |
| Arsenic (As) | ≤0.0005% |
| Bulk Density | 0.9-1.2 |
| Sieve Analysis, % Passing Through | 20 Mesh 100 100 Mesh 8 max. |
| Conclusion | Has been tested and complies with the given specifications |
Package: Bottle, aluminium foil bag, in 25kg composite plastic woven/paper bag with PE liner, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry clean, and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture. Prohibited mixed with hazardous and toxic materials during store and transportation.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.
Chinese Pharmacopoeia 2005 edition included variety: Magnesium Oxide
Name: Magnesium Oxide
Chinese Pinyin: Yanghuamei
English name: Magnesium Oxide
Properties: This product is white powder; Odorless, tasteless; It slowly absorbs carbon dioxide in the air. This product is almost insoluble in water, insoluble in ethanol; Dissolve in dilute acid.
Identification: Identification of magnesium salts in dilute hydrochloric acid solution of this product (Appendix III).
Alkalinity: Take 1.0g of this product, add 50ml of water, boil for 5 minutes, filter while hot, wash the filter residue with appropriate amount of water, the lotion is merged into the filtrate, add several drops of methyl red indicator liquid and 2.0ml of sulfuric acid titration liquid (0.05mol/L), it should be red.
Calcium oxide (CaO): Take about 0.5g of the newly heated and cooled product, weigh it accurately, add 6ml dilute hydrochloric acid, heat and dissolve it, cool it, add 300ml water and 5ml tartric acid solution (1→5), add 10ml trietholamine solution (3→10) and 10ml 45% potassium hydroxide solution, leave it for 5 minutes, add 0.1g calpurhoxin indicator, and then add 0.1g calpurhoxin indicator. The solution was titrated with sodium ethylenediamine tetraacetate (0.olmol /L) until the solution changed from purplish red to blue, and the titration results were corrected with a blank test. Each 1ml of ethylenediamine tetraacetate disodium titrant (0.01mol/L) is equivalent to 0.5608mg of CaO, and the calcium oxide content of this product shall not exceed 0.50%.
Chloride: Take 0.10g of this product, add 0.5ml nitric acid and water to dissolve, dilute with water to 50ml; Separate solution 25ml, check according to law (Appendix Ⅷ A), and compare with the control solution made of standard sodium chloride solution 7.0ml, not more concentrated (0.14%)
Sulphates: Take 0.25g of this product, add 2ml hydrochloric acid and water to dissolve, add water and dilute to 50ml; Separate solution 20ml, check according to law (Appendix Ⅷ B), and compare with the control solution made of standard potassium sulfate solution 3.0ml, not more concentrated (0.3%)
Carbonate: Take 0.10g of this product, add 5ml of water, boil, cool, add 5ml of acetic acid, do not boil. Take 2.0g of this product, add 75ml of water, then add a small amount of hydrochloric acid in batches, stir with addition, until no longer dissolved, boil for 5 minutes, filter, wash the filter residue with water, until the lotion no longer shows the reaction of chloride, incandescent to constant weight, and the residual residue shall not exceed 2.0mg (0.10%).
Loss on Ignition: Take 0.50g of this product, heat to constant weight, weight loss shall not exceed 5.0%.
Iron: Take 50mg of this product, dissolve it with 2ml diluted hydrochloric acid and 23ml water, check according to law (Appendix Ⅷ G), and compare with the control solution made of standard iron solution 25ml, not deeper (0.05%)
Manganese: Take 1.0g of this product, add 20ml of water, 5ml of nitric acid, 5ml of sulfuric acid and 1ml of phosphoric acid, heat and boil for 2 minutes, cool, add 2.0g of potassium periodate, then boil for 5 minutes, cool to room temperature, transfer to 50ml of non-reducible water (add 3ml of nitric acid per 1000ml of water, and boil with 5g potassium periodate for 2 minutes). Dilute to scale, shake well; With standard manganese solution (take anhydrous manganese sulfate 0.275g heated to constant weight at 400-500℃, place in 1000ml measuring bottle, add water to dissolve and dilute to scale, shake well. Each 1ml is equivalent to 0.10mg of Mn) 0.30ml compared with the control solution made by the same method, not deeper (0.003%)
Heavy metals: Take 0.50g of this product, add 10ml diluted hydrochloric acid and 5ml water, heat and dissolve, boil for 1 minute, cool, filter, add 1 drop of phenolphthalic acid indicator liquid to the filtrate, add 2ml of acetate buffer (pH3.5) and appropriate amount of water to make the solution appear light red, add 2ml of acetate buffer (PH3.5) and appropriate amount of water to make 25ml, add 0.5g of ascorbic acid to dissolve. Check according to law (Appendix Ⅷ H first method), 5 minutes of color comparison, containing heavy metals shall not exceed 40 parts per million
Arsenic: Take 0.40g of this product, add 5ml of hydrochloric acid and 23ml of water to dissolve, check according to law (Appendix Ⅷ J, the first method), should meet the regulations (0.0005%).
Assay: Take 0.5g of this product, precision weighing, precision adding sulfuric acid titration solution (0.5mol/L) 30ml dissolved, add 1 drop of methyl orange indicator solution, titration with sodium hydroxide titration solution (1mol/L), according to the amount of sulfuric acid consumed, minus the amount of mixed calcium oxide (CaO) should be consumed, to obtain the amount of sulfuric acid consumed by MgO in the test quantity. Each 1ml of sulphuric acid titrator (0.5mol/L) is equivalent to 20.15mg of MgO or 28.04mg of CaO.
Storage: Keep sealed.
Classification of Chinese and Western medicine: Western medicine (including chemicals, biochemical drugs, antibiotics, radioactive drugs, pharmaceutical excipients)
Chemical component: calculated after ignition to constant weight, containing not less than 96.5% MgO
Molecular formula and molecular weight: MgO 40.30
Pharmacological action: antacid
Magnesium Oxide
MgO 40.30
Magnesium Oxide [1309-48-4]; UNII: 3A3U0GI71G.
DEFINITION
Magnesium Oxide, after ignition, contains NLT 96.0% and NMT 100.5% of Magnesium Oxide (MgO).
IDENTIFICATION
• A. IDENTIFICATION TESTS-GENERAL, Magnesium<191>: A solution in diluted hydrochloric acid meets the requirements.
ASSAY
• PROCEDURE
Diluted ammonium hydroxide: Dilute 67 g of ammonium hydroxide (about 75 mL) with water to 100 mL.
Buffer: Prepare ammonium chloride buffer pH 10 as follows. In a 100-mL volumetric ask, dissolve 5.4 g of ammonium chloride in 20 mL of water, add 35 mL of Diluted ammonium hydroxide, and dilute with water to volume.
Sample stock solution: Ignite a sample of Magnesium Oxide to a constant weight in the temperature range of (800°-900°) ± 25°.
Weigh 320 mg of the ignited sample into a 100-mL volumetric ask, dissolve in 20 mL of 2 N hydrochloric acid, and dilute with water to volume.
Sample solution: Transfer 20.0 mL of the Sample stock solution to a 500-mL ask, and dilute with water to 300 mL. The Sample solution is equivalent to 64 mg of ignited Magnesium Oxide.
Analysis: To the Sample solution, add 10 mL of Buffer and 50 mg of eriochrome black T-sodium chloride indicator. Heat the sample to 40°, and titrate to a blue endpoint with 0.1 M edetate disodium VS. Perform a blank determination, and make any necessary correction to determine the volume of 0.1 M edetate disodium consumed (V ).
Calculate the volume of 0.1 M edetate disodium, Vca , in mL, consumed by calcium, which is present in the portion of Magnesium Oxide taken:
Vca = (W × Lca)/(Fca × 100)
W = amount of ignited Magnesium Oxide in the Sample solution (mg)
Lca = content of calcium as determined in the test for Limit of Calcium (%)
Fca = weight of calcium equivalent to each mL of 0.1 M edetate disodium, 4.008 mg
Calculate the percentage of Magnesium Oxide (MgO) in the portion of Magnesium Oxide taken:
Result = (Vs − Vca ) × (FMgO /W) × 100
Vs = volume of 0.1 M edetate disodium consumed by the Sample solution (mL)
Vca = volume of 0.1 M edetate disodium consumed by calcium (mL)
FMgO = weight of Magnesium Oxide equivalent to each mL of 0.1 M edetate disodium, 4.030 mg
W = amount of the ignited Magnesium Oxide in the Sample solution (mg)
Acceptance criteria: 96.0%-100.5% after ignition
IMPURITIES
• FREE ALKALI AND SOLUBLE SALTS
Sample solution: Boil 2.0 g with 100 mL of water for 5 min in a covered beaker, and lter while hot. Allow to cool, and dilute with water to 100 mL.
Analysis 1: To 50 mL of the Sample solution add methyl red TS, and titrate with 0.10 N sulfuric acid.
Acceptance criteria 1: NMT 2.0 mL of the acid is consumed.
Analysis 2: Evaporate 25 mL of the remaining Sample solution to dryness, and dry at 105° for 1 h.
Acceptance criteria 2: NMT 10 mg of residue remains (NMT 2.0%).
• ACID-INSOLUBLE SUBSTANCES
Sample: 2 g
Analysis: Mix the Sample with 75 mL of water, add hydrochloric acid in small portions with agitation until no more dissolves, and boil for 5 min. If an insoluble residue remains, lter, wash well with water until the last washing is free from chloride, and ignite.
Acceptance criteria: The weight of the ignited residue is NMT 2 mg (NMT 0.1%).
• LIMIT OF CALCIUM
[NOTE-A commercially available atomic absorption standard solution for calcium may be used where preparation of a calcium standard stock solution is described below. Concentrations of the Standard solutions and the Sample solution may be modied to t the linear or working range of the instrument.]
Dilute hydrochloric acid: Dilute 100 mL of hydrochloric acid with water to 1000 mL.
Lanthanum solution: To 58.65 g of lanthanum oxide, add 400 mL of water, and add, gradually with stirring, 250 mL of hydrochloric acid. Stir until dissolved, and dilute with water to 1000 mL.
Standard solutions: Transfer 249.7 mg of calcium carbonate, previously dried at 300° for 3 h and cooled in a desiccator for 2 h, to a 100-mL volumetric ask. Dissolve in a minimum amount of hydrochloric acid, and dilute with water to volume. Transfer 1.0, 5.0, 10.0, and 15.0 mL of this stock solution to separate 1000-mL volumetric asks, each containing 20 mL of the Lanthanum solution and 40 mL of Dilute hydrochloric acid, and dilute with water to volume. These Standard solutions contain 1.0, 5.0, 10.0, and 15.0 µg/mL of calcium, respectively.
Sample solution: Transfer 250 mg of Magnesium Oxide, freshly ignited for 1 h in the temperature range of (800°–900°) ± 25°, to a beaker. Add 30 mL of Dilute hydrochloric acid, and stir until dissolved, heating if necessary. Transfer the solution so obtained to a 200-mL volumetric ask containing 4 mL of Lanthanum solution, and dilute with water to volume.
Blank solution: Transfer 4 mL of the Lanthanum solution and 10 mL of Dilute hydrochloric acid to a 200-mL volumetric ask, and dilute with water to volume.
Instrumental conditions
(See Atomic Absorption Spectroscopy <852>.)
Mode: Atomic absorption spectrophotometry
Analytical wavelength: Calcium emission line at 422.7 nm
Lamp: Calcium hollow-cathode
Flame: Nitrous oxide–acetylene
Analysis
Samples: Standard solutions, Sample solution, and Blank solution
Using the calibration graph, determine the concentration, C, in µg/mL, of calcium in the Sample solution.
Calculate the percentage of calcium in the portion of Magnesium Oxide taken:
Result = [(V/W) × C × F] × 100
V = volume of the Sample solution (mL)
W = weight of Magnesium Oxide (mg)
C = concentration of calcium in the Sample solution (µg/mL)
F = conversion from µg/mL to mg/mL, 0.001
Acceptance criteria: NMT 1.1%
• IRON <241>
Test preparation: Boil 40 mg with 5 mL of 2 N nitric acid for 1 min. Cool, dilute with water to 50 mL, and mix. Dilute 25 mL of this
solution with water to 45 mL, and add 2 mL of hydrochloric acid.
Acceptance criteria: NMT 0.05%
SPECIFIC TESTS
• LOSS ON IGNITION <733>
Sample: 500-1000 mg
Analysis: Transfer the Sample to a tared platinum crucible, and ignite in the temperature range of (800°-900°) ± 25° to constant weight.
Acceptance criteria: NMT 10.0%
• BULK DENSITY AND TAPPED DENSITY OF POWDERS, Bulk Density, Method I<616>: Using the procedure specied in the chapter, determine the bulk density of Magnesium Oxide.
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in tight containers.
• LABELING: Label it to indicate its bulk density. The indicated density may be in the form of a range
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| Safety Description | S24/25 - Avoid contact with skin and eyes |
| UN IDs | UN 1418 |
| HS Code | 2519909100 |
1. Heating elements. Magnesium Oxide (MgO) is as a refractory material.MgO is used as a basic refractory material for crucibles.
2. Fireproofing. As a construction material, Magnesium Oxide wallboards have several attractive characteristics: fire resistance, termite resistance, moisture resistance, mold and mildew resistance, and strength.
3. In the pharmaceutical industry. Magnesium Oxide is used for relief of heartburn and indigestion, as an antacid, magnesium supplement, and as a short-term laxative. It is also used to improve symptoms of indigestion. Side effects of magnesium oxide may include nausea and cramping.
4. As a food additive, in the food industry, Magnesium Oxide is used as an anticaking agent, used as a flour improver, nutrient fortifier, processing aid, regulator, etc. As an anti-caking agent and anti-acid agent used in wheat flour, milk powder chocolate, cocoa powder, grape powder, sugar powder and other fields,
5. In the plastics industry as fillers.
6. Also can be used in the manufacture of ceramics, enamel, glass, dye and other fields.
7. As a filling material in the manufacture of polishing agents, adhesives, and paints.
8. As a promoter and catalyst in rayon, rubber (chloroprene rubber, fluororubber).