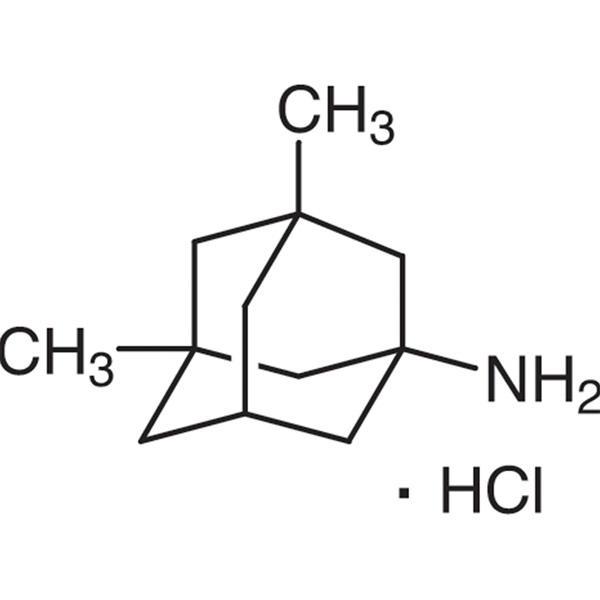
Memantine Hydrochloride Memantine HCl CAS 41100-52-1 Assay 99.0%~101.0% API
Manufacturer with High Purity and Stable Quality
Chemical Name: Memantine Hydrochloride
CAS: 41100-52-1
Memantine Hydrochloride in the treatment of Alzheimer's disease
API USP Standard, High Quality, Commercial Production
| Chemical Name | Memantine Hydrochloride |
| Synonyms | Memantine HCl; 3,5-Dimethyl-1-adamantanamine Hydrochloride |
| CAS Number | 41100-52-1 |
| CAT Number | RF-API43 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C12H22ClN |
| Molecular Weight | 215.76 |
| Melting Point | 292℃ |
| Shipping Condition | Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Crystal Powder |
| Identification | IR |
| Loss on Drying | ≤0.50% |
| Heavy Metals (Pb) | ≤10ppm |
| Related Substances | |
| 1-Methyladamantane | ≤0.30% (CAS 768-91-2) |
| 1,3,5-Trimethyladamantane | ≤0.30% (CAS 707-35-7) |
| Any Unknow Impurity | ≤0.10% |
| Total Impurities | ≤0.50% |
| Residual Solvent | Ethanol ≤0.05% |
| Residual Solvent | Ethylacetate ≤0.05% |
| Contamination Microbiologica | Max 5*102 aerobes and fungi per 1g No Escherichia coli |
| Size of Distributed Particles | Pass 100um |
| pH | 4.5~6.5 |
| Assay | 99.0%~101.0% (on the dried basis) |
| Test Standard | USP Standard |
| Usage | Active Pharmaceutical Ingredient (API); Alzheimer’s Disease |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Memantine Hydrochloride (CAS: 41100-52-1) with high quality.
Memantine Hydrochloride (CAS: 41100-52-1) is a well- known neuroprotective drug used for the treatment of Alzheimer’s Disease. It is believed to be the first neuroprotective drug that has achieve clinical approval by US FDA as well as European.
Memantine Hydrochloride, a NMDA receptor antagonist, was co-developed by Forest Laboratories with Merz Pharmaceuticals and marketed under the trade name Namenda for the treatment of Alzheimer’s disease in the US after its approval in October, 2003. This drug has been available in many European and Asian markets before getting approval in the US.
-
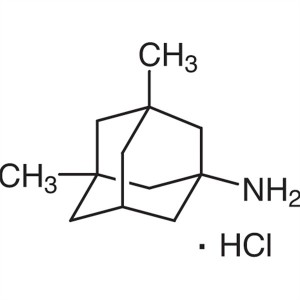
Memantine Hydrochloride Memantine HCl CAS 41100...
-
CAS 665-66-7 Assay 98.5%~101.5% API
-
Rimantadine Hydrochloride CAS 1501-84-4 Purity ...
-
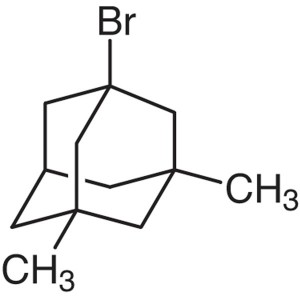
1-Bromo-3,5-Dimethyladamantane CAS 941-37-7 Pur...
-
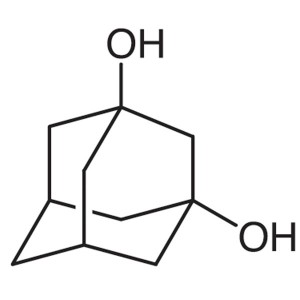
1,3-Adamantanediol CAS 5001-18-3 Purity >99.0% ...
-
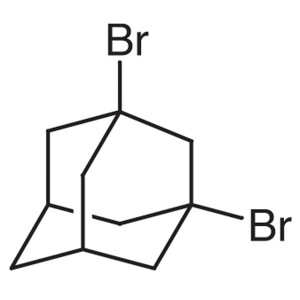
1,3-Dibromoadamantane CAS 876-53-9 Purity >99.0...
-
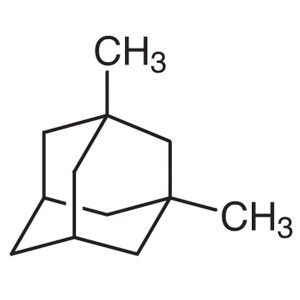
1,3-Dimethyladamantane CAS 702-79-4 Purity >99....
-
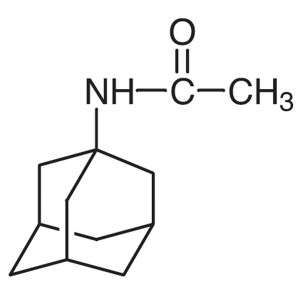
1-Acetamidoadamantane CAS 880-52-4 Purity >99.0...
-
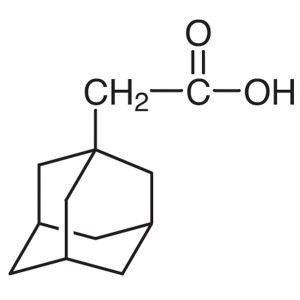
1-Adamantaneacetic Acid CAS 4942-47-6 Purity >9...
-
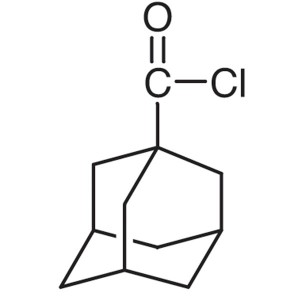
1-Adamantanecarbonyl Chloride CAS 2094-72-6 Pur...
-
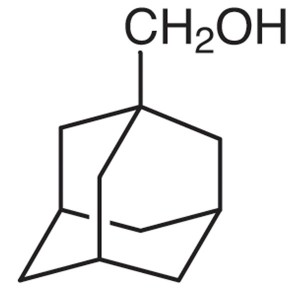
1-Adamantanemethanol CAS 770-71-8 Purity >99.0%...
-
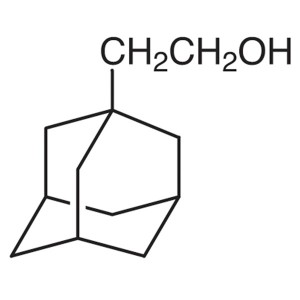
1-Adamantaneethanol CAS 6240-11-5 Purity >98.0%...
-
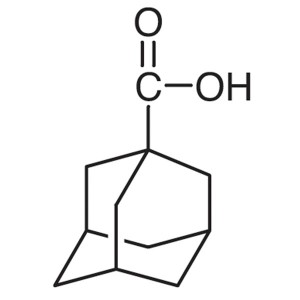
1-Adamantanecarboxylic Acid CAS 828-51-3 Purity...
-
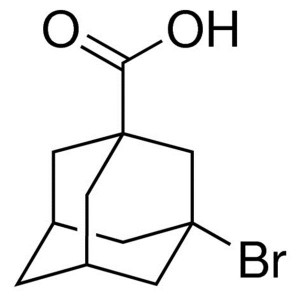
3-Bromoadamantane-1-Carboxylic Acid CAS 21816-0...
-
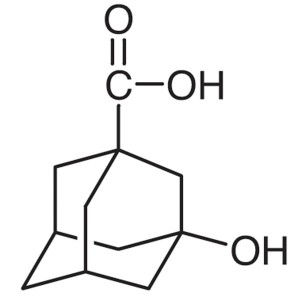
3-Hydroxy-1-Adamantanecarboxylic Acid CAS 42711...
-
Adamantane CAS 281-23-2 Purity >99.0% (GC)