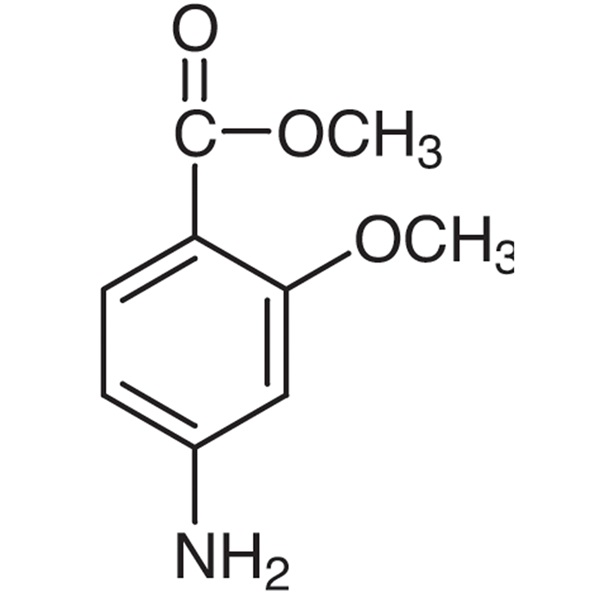
Methyl 4-Amino-2-Methoxybenzoate CAS 27492-84-8 Purity >98.0% (GC) Lenvatinib Mesylate Intermediate Factory
Ruifu Chemical Supply Lenvatinib Mesylate Intermediates With High Purity
Lenvatinib Mesylate CAS 857890-39-2
4-Chloro-7-Methoxyquinoline-6-Carboxamide CAS 417721-36-9
Desquinolinyl Lenvatinib; 1-(2-Chloro-4-Hydroxyphenyl)-3-Cyclopropylurea CAS 796848-79-8
Methyl 7-Methoxy-4-Oxo-1,4-Dihydroquinoline-6-Carboxylate CAS 205448-65-3
Methyl 4-Amino-2-Methoxybenzoate CAS 27492-84-8
5-(Methoxymethylene)-2,2-Dimethyl-1,3-Dioxane-4,6-Dione CAS 15568-85-1
4-Amino-3-Chlorophenol CAS 17609-80-2
4-Amino-3-Chlorophenol Hydrochloride CAS 52671-64-4
Methyl 4-Chloro-7-Methoxyquinoline-6-Carboxylate CAS 205448-66-4
| Chemical Name | Methyl 4-Amino-2-Methoxybenzoate |
| Synonyms | 4-Amino-2-Methoxybenzoic Acid Methyl Ester; Methyl 4-Amino-o-Anisate; 4-Amino-o-Anisic Acid Methyl Ester |
| CAS Number | 27492-84-8 |
| CAT Number | RF-PI1974 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C9H11NO3 |
| Molecular Weight | 181.19 |
| Density | 1.179±0.060 g/cm3 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder or Crystals |
| Purity / Analysis Method | >98.0% (GC) |
| Purity / Analysis Method | 97.5~102.5 (Nonaqueous Titration) |
| Melting Point | 157.0~161.0℃ |
| Loss on Drying | <1.00% |
| Total Impurities | <2.0% |
| Infrared Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Lenvatinib Mesylate (CAS: 857890-39-2) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Methyl 4-Amino-2-Methoxybenzoate (CAS: 27492-84-8) can be used as an intermediate of Lenvatinib Mesylate (CAS: 857890-39-2). Lenvatinib is a thyroid cancer drug developed by Eisai Corporation of Japan (Code: E7080), belonging to the inhibitor of oral multi-receptor tyrosine kinase (RTK) and can inhibit the kinase activity of the vascular endothelial growth factor (VEGF) Receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). Lenvatinib can also inhibit the involvement of other RTKs in pathological angiogenesis, tumor growth, and cancer progression except for their normal cellular functions including fibroblast growth factor (FGF) receptors FGFR1, 2, 3, and 4; platelet-derived growth factor receptor (PDGFR [alpha]), KIT, and RET. [Indications]: Lenvatinib is suitable for the treatment of patients of thyroid cancer of local recurrence or metastasis type, progressivity type and radioactive iodine-refractory differentiated type. On February 13, 2015, the US FDA approved anticancer drug Lenvatinib for the treatment of thyroid cancer. Lenvatinib is a multi-target enzyme inhibitor, being capable of inhibiting the VEGFR2 and VEGFR3 (vascular endothelial growth factor receptor). The trade name of Lenvatinib is Lenvima. On May 20, 2015, the European Medicines Agency (EMA) approved Lenvatinib for the treatment of invasive, locally advanced or metastatic differentiated (papillary, follicular, Hurthle type) thyroid cancer (DTC). In the trial, the median survival time for patients of radioactive iodine-refractory DTC treated with Lenvatinib was 18 months while the value for patients who take placebo is only 3 months.
-
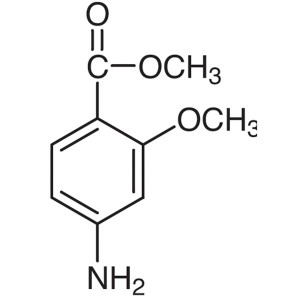
Methyl 4-Amino-2-Methoxybenzoate CAS 27492-84-8...
-
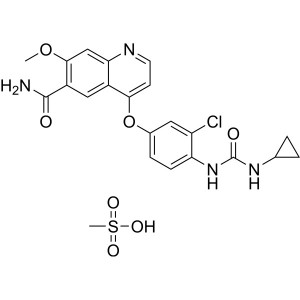
Lenvatinib Mesylate CAS 857890-39-2 Assay 98.0~...
-
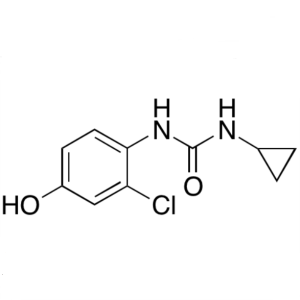
Desquinolinyl Lenvatinib CAS 796848-79-8 Purity...
-
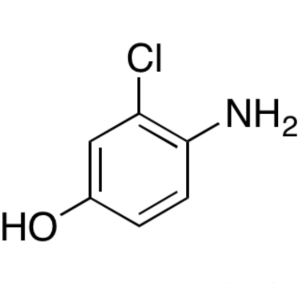
4-Amino-3-Chlorophenol CAS 17609-80-2 Lenvatini...
-
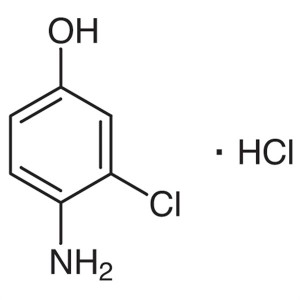
4-Amino-3-Chlorophenol Hydrochloride CAS 52671-...
-
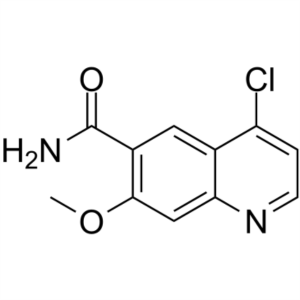
4-Chloro-7-Methoxyquinoline-6-Carboxamide CAS 4...
-
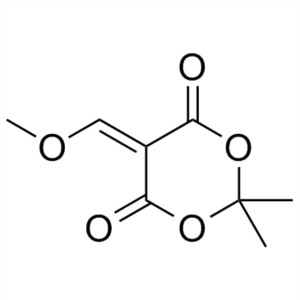
Lenvatinib Mesylate Intermediate CAS 15568-85-1...
-
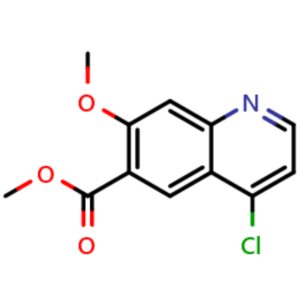
Methyl 4-Chloro-7-Methoxyquinoline-6-Carboxylat...
-
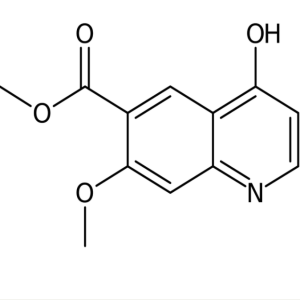
Lenvatinib Mesylate Intermediate CAS 205448-65-...