Molnupiravir (EIDD-2801) CAS 2349386-89-4 COVID-19 API High Quality
Commercial Supply Molnupiravir and Related Intermediates with High Quality
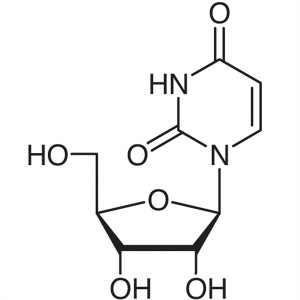
Uridine CAS 58-96-8
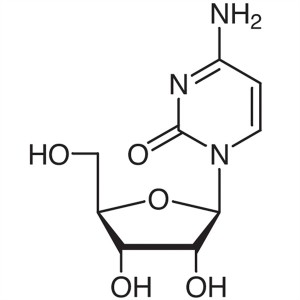
Cytidine CAS 65-46-3
Molnupiravir N-1 CAS 2346620-55-9
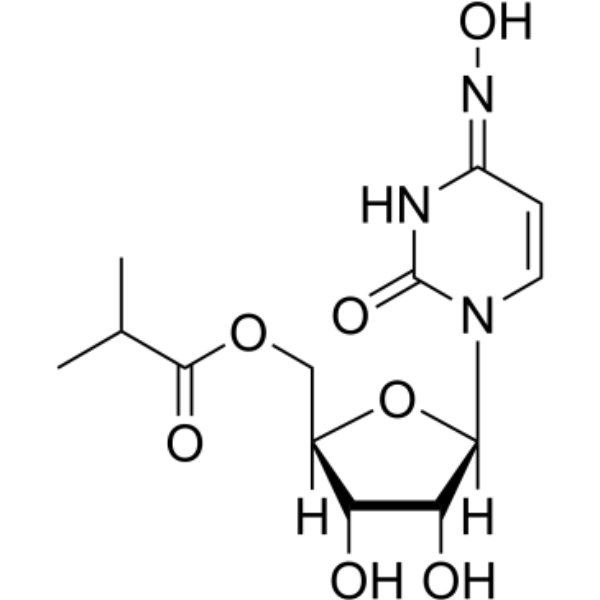
Molnupiravir (EIDD-2801) CAS 2349386-89-4
| Chemical Name | Molnupiravir (EIDD-2801) |
| Synonyms | MK-4482; β-D-N4-Hydroxycytidine-5′-isopropyl ester; ((2R,3S,4R,5R)-3,4-dihydroxy-5-((E)-4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl isobutyrate |
| CAS Number | 2349386-89-4 |
| CAT Number | RF-API97 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C13H19N3O7 |
| Molecular Weight | 329.31 |
| Solubility | Soluble in DMSO |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Identification IR | Sample Spectrum corresponds to that of reference standard |
| Identification HPLC | The retention time of the major peak of the sample solution corresponds to that of the standard solution |
| Related Substances | |
| Impurity A | ≤0.15% |
| Impurity B | ≤0.15% |
| Any Unspecified Impurity | ≤0.15% |
| Total Unspecified Impurities | ≤0.30% |
| Total Impurities | ≤0.50% |
| Residual Solvents | |
| N-Heptane | ≤5000ppm |
| Ethanol | ≤5000ppm |
| Isopropyl Acetate | ≤5000ppm |
| Acetonitrile | ≤410ppm |
| Methylene Dichloride | ≤600ppm |
| Acetone | ≤5000ppm |
| Isopropanol | ≤5000ppm |
| Water Content (K.F) | ≤0.50% |
| Residue on Ignition | ≤0.10% |
| Optical Rotation | -7.5° to -9.5° (C=0.5, Methanol) |
| Heavy Metals | ≤10ppm |
| Purity / Analysis Method | ≥99.5% (230nm) |
| Assay / Analysis Method | 98.0%~102.0% (HPLC on dried basis) |
| Shelf Life | 24 Months |
| Test Standard | Enterprise Standard |
| Usage | API, Molnupiravir (EIDD-2801) COVID-19 Inhibitor |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Molnupiravir (EIDD-2801, MK-4482) is an orally bioavailable prodrug of the ribonucleoside analog β-d-N4-hydroxycytidine (NHC; EIDD-1931) with broad-spectrum antiviral activity against SARS-CoV-2, MERS-CoV, SARS-CoV, and the causative agent of COVID-19. Molnupiravir is sold under the brand name Lagevrio and generically as emorivir. Molnupiravir has been shown to improve pulmonary function, decrease body weight loss and reduce the amount of virus in the lung. In addition to activity against coronaviruses, Molnupiravir, in laboratory studies, has demonstrated activity against seasonal and bird influenza, respiratory syncytial virus, chikungunya virus, Ebola virus, Venezuelan equine encephalitis virus, and Eastern equine encephalitis virus. Molnupiravir was originally developed to treat influenza at Emory University by the university's drug innovation company, Drug Innovation Ventures at Emory (DRIVE), but was reportedly abandoned for mutagenicity concerns. It was then acquired by Miami-based company Ridgeback Biotherapeutics, which later partnered with Merck & Co. to develop the drug further. Molnupiravir was approved for medical use in the United Kingdom in November 2021.