Newly Arrival Irinotecan Hydrochloride Trihydrate - Favipiravir CAS 259793-96-9 T-705 COVID-19 API High Quality – Ruifu
Newly Arrival Irinotecan Hydrochloride Trihydrate - Favipiravir CAS 259793-96-9 T-705 COVID-19 API High Quality – Ruifu Detail:
Manufacturer with High Purity and Stable Quality
Commercial Supply Favipiravir (CAS: 259793-96-9) and Related Intermediates:
Favipiravir CAS: 259793-96-9
3,6-Dichloropyrazine-2-Carbonitrile CAS: 356783-16-9
3,6-Difluoropyrazine-2-Carbonitrile CAS: 356783-28-3
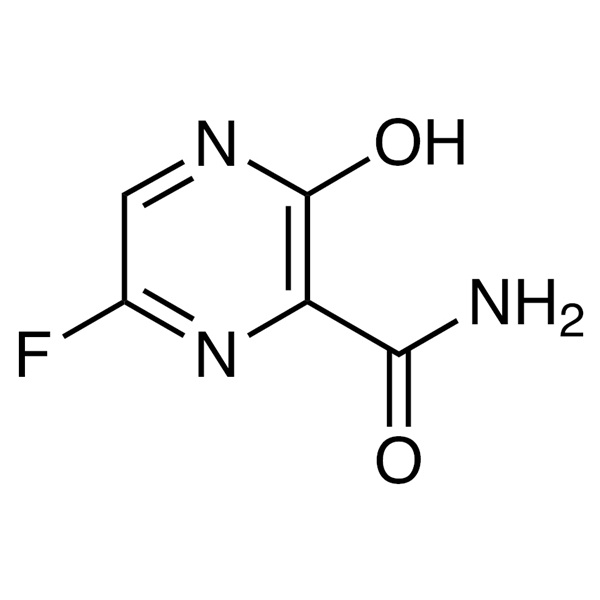
6-Bromo-3-Hydroxypyrazine-2-Carboxamide CAS: 259793-88-9
3-Hydroxypyrazine-2-Carboxamide CAS: 55321-99-8
2-Aminopropanediamide CAS: 62009-47-6
Diethyl Aminomalonate Hydrochloride CAS: 13433-00-6
| Chemical Name | Favipiravir |
| Synonyms | T-705; 6-Fluoro-3-Hydroxy-2-Pyrazinecarboxamide |
| CAS Number | 259793-96-9 |
| CAT Number | RF-API18 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C5H4FN3O2 |
| Molecular Weight | 157.1 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Off-White Powder |
| Identification | 1) IR 2) HPLC |
| Melting Point | 188.0℃-193.0℃ |
| Related Substances (Area Normalization) |
Any Single Impurity: ≤0.10% (HPLC) |
| Total Impurities: ≤1.0% (HPLC) | |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Moisture (K.F) | ≤0.50% |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤20ppm |
| Residue Solvents | |
| Methanol | ≤3000ppm |
| Ethyl Acetate | ≤5000ppm |
| Isopropanol | ≤5000ppm |
| Acetonitrile | ≤410ppm |
| n-Heptane | ≤5000ppm |
| Test Standard | Enterprise Standard |
| Usage | Active Pharmaceutical Ingredient (API); Treatment of COVID-19 |
Package: Bottle, Aluminum foil bag, 25kg/Cardboard drum, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Favipiravir (T-705) (CAS 259793-96-9) is one of the 5 compounds recommended by WHO for the investigation of treatment of COVID-19.
Favipiravir (T-705) is a selective inhibitor of viral RNA-dependent RNA polymerase with activity against many RNA viruses, influenza viruses, West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses. Favipiravir is a broad-spectrum antiviral drug that was cleared by the Drugs Controller General of India (DCGI) last week for “emergency restricted” use among Covid-19 patients. Favipiravir was originally developed in the late 1990s by a company that was later purchased by the Japanese firm Fujifilm as part of its transition from the photo business to healthcare. After being tested against a range of viruses, the drug was approved in Japan in 2014 for emergency use against flu epidemics or to treat new strains of influenza.
Product detail pictures:
Related Product Guide:
We continue to keep increasing and perfecting our solutions and service. At the same time, we operate actively to do research and enhancement for Newly Arrival Irinotecan Hydrochloride Trihydrate - Favipiravir CAS 259793-96-9 T-705 COVID-19 API High Quality – Ruifu , The product will supply to all over the world, such as: Ottawa, Egypt, Luxembourg, We confirm to public, cooperation, win-win situation as our principle, adhere to the philosophy of make a living by quality, keep developing by honesty , sincerely hope to build up a good relationship with more and more customers and friends, to achieve a win-win situation and common prosperity.
-
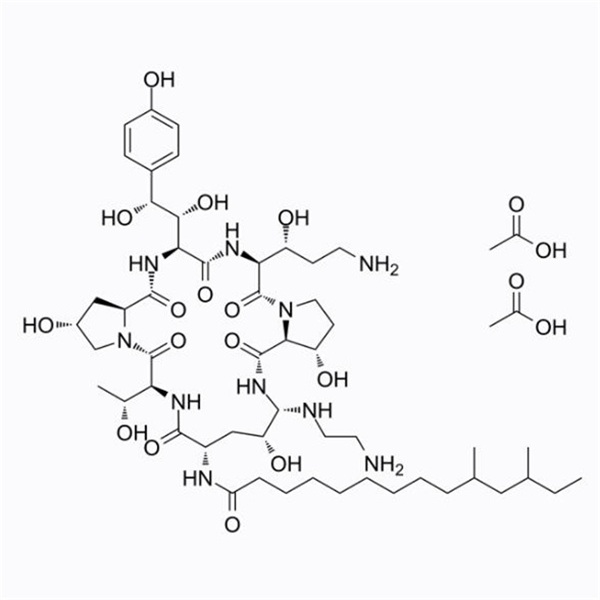
New Delivery for Pregabalin - Caspofungin Acet...
-
Hot Selling for Deoxyadenosine - N-Methyl-4-Ni...
-
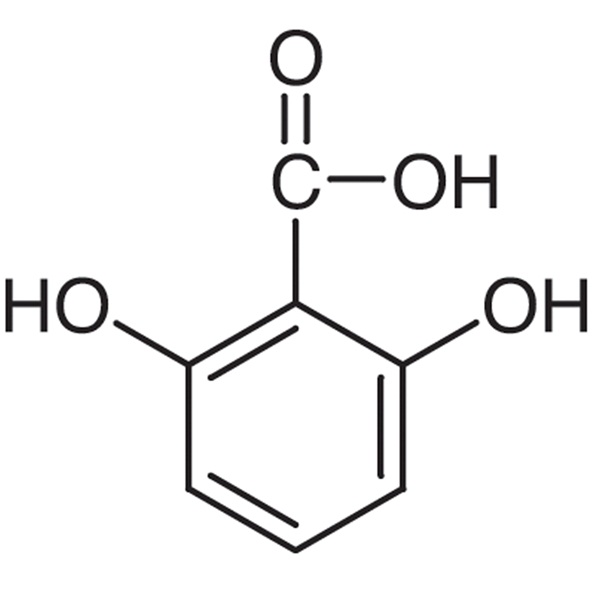
Cheap PriceList for 6-KT - 2,6-Dihydroxybenzoi...
-
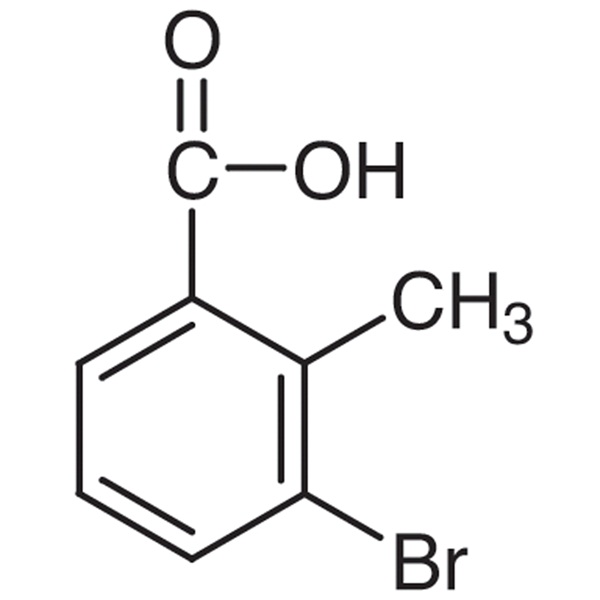
High Quality for Acetic Acid Isopropenyl Ester ...
-
China Supplier (S)-4-Benzyl-2-Oxazolidinone - ...
-
Massive Selection for 1-Phenyl-1 2 3 4-tetrahyd...
Reasonable price, good attitude of consultation, finally we achieve a win-win situation,a happy cooperation!
