Newly Arrival Irinotecan Hydrochloride Trihydrate - (R)-Lansoprazole Dexlansoprazole CAS 138530-94-6 Assay 98.0~102.0% (HPLC) Factory – Ruifu
Newly Arrival Irinotecan Hydrochloride Trihydrate - (R)-Lansoprazole Dexlansoprazole CAS 138530-94-6 Assay 98.0~102.0% (HPLC) Factory – Ruifu Detail:
Ruifu Chemical Supply Lansoprazole Intermediates
Lansoprazole CAS 103577-45-3
Lansoprazole Chloride Compound CAS 127337-60-4
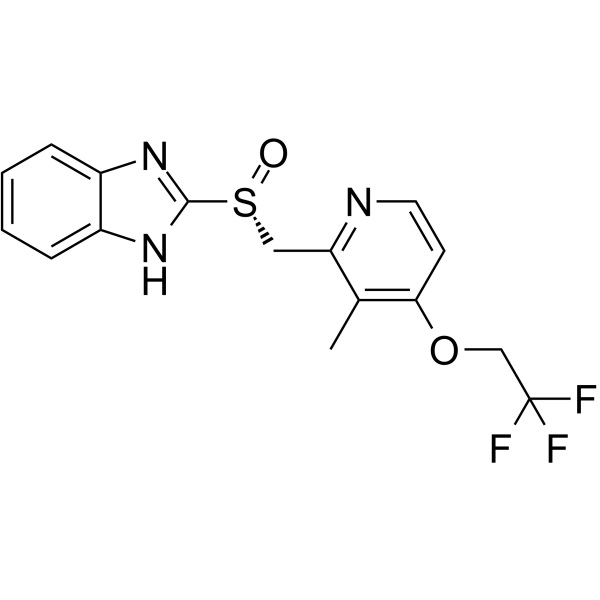
(R)-Lansoprazole Dexlansoprazole CAS 138530-94-6
| Chemical Name | (R)-Lansoprazole |
| Synonyms | R-(+)-Lansoprazole; Dexlansoprazole; 2-[(R)-[[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole; T 168390; TAK 390; Lansoprazole Impurity 14; Dexlansoprazole Related Impurities 2; (R)-2-[[[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]-1H-Benzimidazole; |
| CAS Number | 138530-94-6 |
| CAT Number | RF-PI1916 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C16H14F3N3O2S |
| Molecular Weight | 369.36 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Solubility | Soluble in Dimethylformamide, Almost Insoluble in Water |
| Clarity&Colour of Solution | Meets the Requirement |
| Identification IR | The infrared absorption spectrum should be consistent with the reference substance |
| Identification HPLC | The retention time of the main peak should be consistent with that of the reference |
| Specific Rotation | +142.0°~+149.0° |
| Melting Point | ~140.0℃ |
| Water Content (K.F) | <1.00% |
| Residue on Ignition | <0.10% |
| Related Impurities | (HPLC) |
| Nitrogen Oxides | <0.10% |
| Sulfone | <0.40% |
| Sulfide | <0.20% |
| Any Other Single Impurity | <0.10% |
| Total Impurities | <0.60% |
| Optical Purity (HPLC) | >99.5% |
| Assay | 98.0~102.0% (HPLC, %w/w, on anhydrous basis, solvent-free) |
| Heavy Metals | <10ppm |
| Residual Solvents | |
| Toluene | <890ppm |
| n-Heptane | <5000ppm |
| Bacterial Endotoxin | <2.5EU/mg |
| Test Standard | USP Standard; Chinese Pharmacopoeia |
| Usage | API; An Orally Active Proton Pump Inhibitor |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer’s requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


(R)-Lansoprazole (CAS: 138530-94-6) is the R enantiomer of Lansoprazole, Lansoprazole (AG 1749) is an orally active proton pump inhibitor which prevents the stomach from producing acid. Dexlansoprazole for Lansoprazole dextroisomer, is a kind of anti peptic ulcer drugs for substituted benzimidazole derivatives, in the molecular structure into the element fluorine, second proton pump inhibitors omeprazole after. Dexlansoprazole has the effect of inhibiting the secretion of gastric acid, the effect is better than that of other drugs (Omeprazole, Pantoprazole, Rebela, Tetrazolium) can significantly inhibit the occurrence of ulcer. It is superior to famotidine or Omeprazole for the damage of alcohol induced gastric mucosa and duodenal ulcer mainly caused by acid hypersecretion. In addition, the product has the same anti Helicobacter pylori effect as the bismuth preparation, and is used for reflux esophagitis and Zhuo AI syndrome. Takeda Pharmaceuticals received approval of dexlansoprazole, a dual release formulation of the (R)-isomer of lansoprazol proton pump inhibitor (PPI) already in the market, from the FDA in January 2009. Dexlansoprazole is a delayed release capsule for the oncedaily, oral treatment of heartburn associated with symptomatic non-erosive gastroesophageal reflux disease (GERD), the healing of erosive esophagitis (EE) and the maintenance of healed EE.
Product detail pictures:
Related Product Guide:
Using a complete scientific high quality management program, superior high quality and superior faith, we acquire great reputation and occupied this industry for Newly Arrival Irinotecan Hydrochloride Trihydrate - (R)-Lansoprazole Dexlansoprazole CAS 138530-94-6 Assay 98.0~102.0% (HPLC) Factory – Ruifu , The product will supply to all over the world, such as: Denver, Puerto Rico, South Korea, We are looking forward to establishing a mutually beneficial relationship with you based on our high-quality products, reasonable prices and best service. We hope that our products will bring you a pleasant experience and carry a feeling of beauty.
-
OEM Factory for USP Standard - 3-Hydroxypyridi...
-
Free sample for Citicoline - 3,5-Dibromopyridi...
-
China Factory for (S)-3-Pyrrolidinol Hydrochlor...
-
Wholesale Dealers of R-Benzyl Glycidyl Ether -...
-
Hot-selling Ethyl (R)-(+)-4-Chloro-3-Hydroxybut...
-
Special Design for Phenylethyl Alcohol - (S)-(...
The product classification is very detailed that can be very accurate to meet our demand, a professional wholesaler.
