Newly Arrival Irinotecan Hydrochloride Trihydrate - Temozolomide (TMZ) CAS 85622-93-1 Assay 99.0%~101.0% API Factory High Purity – Ruifu
Newly Arrival Irinotecan Hydrochloride Trihydrate - Temozolomide (TMZ) CAS 85622-93-1 Assay 99.0%~101.0% API Factory High Purity – Ruifu Detail:
Manufacturer with High Purity and Stable Quality
Supply Temozolomide and Related Intermediates:
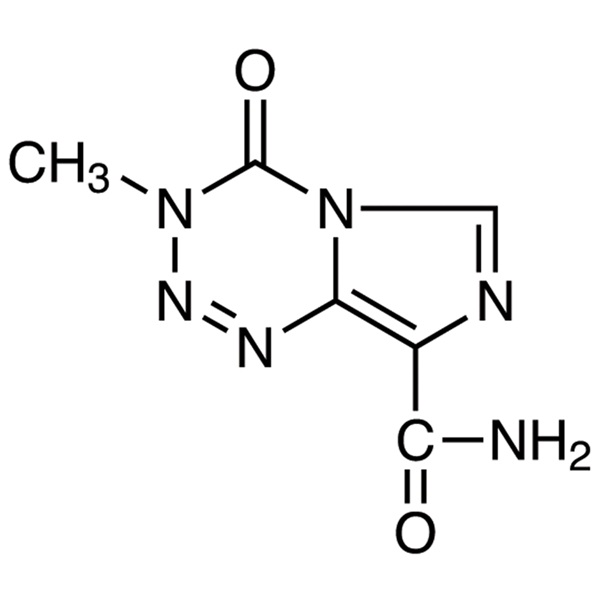
Temozolomide CAS: 85622-93-1
5(4)-Amino-4(5)-imidazolecarboxamide Hydrochloride CAS: 72-40-2
| Chemical Name | Temozolomide |
| Synonyms | 3,4-Dihydro-3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide |
| CAS Number | 85622-93-1 |
| CAT Number | RF-API29 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H6N6O2 |
| Molecular Weight | 194.15 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or to Light Pink Powder |
| Dimethyl Sulphoxide | ≤0.50% |
| Related Substances | |
| The Known Impurity | AIC≤0.10% |
| Single Impurity | ≤0.10% |
| Rest Related Impurities | ≤0.30% |
| Total Impurities | ≤0.30% |
| Heavy Metals | ≤10ppm |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.10% |
| Assay | 99.0%~101.0% |
| Test Standard | Enterprise Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Temozolomide (CAS: 85622-93-1) with high quality, active pharmaceutical ingredient (API). Temozolomide (TMZ) is an oral alkylating agent used to treat Glioblastoma Multiforme (GBM) and Astrocytomas, Metastatic Melanoma.
Temozolomide is the first effective orally-taken imidazole and tetrazine-class anticancer drug which belongs to the second generation of an alkylating agent with antitumor activity without liver metabolic activation after oral administration. It is characterized by easily penetration through the blood-brain barrier, good tolerance and being not superimposed with other drugs toxicity, and having synergistic effect with radiotherapy which is suitable for treating malignant glioma recurrence after conventional treatment such as glioblastoma multiforme tumors or degenerative astrocytoma. It is first-line drug for treatment of metastatic melanoma.
Temozolomide had been first synthesized by Cancer Research UK Group, and then be transferred to the Schering-Plough Company (United States) for development. The drug is different from the existing Antineoplastic drug. It has a novel chemical structure and belongs to a four-imidazole derivative. Temozolomide does not play a direct role. At physiological pH, it is first quickly converted into active compound MTIC [5-(3-methyl-triazene-1-) imidazole-4-carboxamide] via non-enzymatically way. People think that the cytotoxicity of MTIC is mainly due to its DNA alkylation (methylation) effect. Alkylation mainly occurs in the O6 and N7 position of guanine. Basic and clinical studies of temozolomide have confirmed that it is effective in treating some of the most common glioma cells. In 1999, it has been approved for enter into market in EU and the US wherein the permitted indication in United States is mainly for second-line treatment of glioblastoma multiforme and degenerative star gliomas and approved indications of EU is for treating developing or relapsing glioblastoma multiforme which has already been subject to conventional therapy. The efficacy of temozolomide on treating glioblastoma multiforme has received more recognition in Europe.
Product detail pictures:
Related Product Guide:
The corporate keeps towards the operation concept "scientific administration, superior quality and performance primacy, client supreme for Newly Arrival Irinotecan Hydrochloride Trihydrate - Temozolomide (TMZ) CAS 85622-93-1 Assay 99.0%~101.0% API Factory High Purity – Ruifu , The product will supply to all over the world, such as: Bulgaria, Lithuania, Bulgaria, When It produced, it making use of the world's major method for reliable operation, a low failure price, it appropriate for Jeddah shoppers choice. Our enterprise. s situated inside the national civilized cities, the website traffic is very hassle-free, unique geographical and financial circumstances. We pursue a "people-oriented, meticulous manufacturing, brainstorm, make brilliant" company philosophy. Strict good quality management, fantastic service, affordable cost in Jeddah is our stand around the premise of competitors. If needed, welcome to make contact with us by our web page or phone consultation, we will be delighted to serve you.
-
8 Year Exporter ECPPA - Atorvastatin tert-Buty...
-
Factory wholesale Lapatinib Intermediate - 4-M...
-
Leading Manufacturer for Sirolimus - Ezetimibe...
-
OEM Customized Methyl (S)-(-)-2-Chloropropionat...
-
Reasonable price (S)-3-Amino-3-phenyl-1-propano...
-
Factory wholesale Sofosbuvir Intermediate - 2-...
The quality of the products is very good, especially in the details, can be seen that the company work actively to satisfy customer's interest, a nice supplier.
