
The 3rd CMC-China 2021
Time: September 29-30, 2021
Exhibition Venue: C-D Hall, Suzhou International Expo Center, Suzhou City, JIangsu Province, China.
Synergy of Centralized China’s Drug Volume-based Purchasing and Medical Insurance Negotiation Policy intensifies the pain of pharmaceutical industrial integration and improves the quality of generic drugs.The evaluation and approval of new drugs was accelerated, the price of medical insurance negotiation was reduced, and featured drugs were removed from medical insurance, freeing up more space for drugs with real clinical application value: NMPA joining ICH and the implementation of MAH system increased the speed of industrialization of innovative pharmaceutical projects.
For quite a period of time, the pharmaceutical industry focused on "innovation" and ignored the drug demand in line with national conditions and the whole pharmaceutical industry chains, such as the integration demand of API intermediates and supply chain;Or the marketing channel of the innovative drugs.Innovation for innovation's sake is a castle in the air.
How should the pharmaceutical industry with Chinese characteristics go in the future?This is not only a problem on the pharmaceutical R&D side, but also that the whole pharmaceutical industry chain needs to think about.
September 29 to 30, 2021, the third China Chemical and Biological Pharmaceutical Industry Conference will be held in Suzhou International Expo Center.The theme of the conference is "Linking the whole pharmaceutical industry chain to solve the unmet drug demand in line with China's national conditions", At the conference, about 6000~8000 participants will discuss the API intermediates, pathway inhibitor,and improvement and innovation, then to the source of innovative new drugs in all directions to explore the technology, policies and solutions.
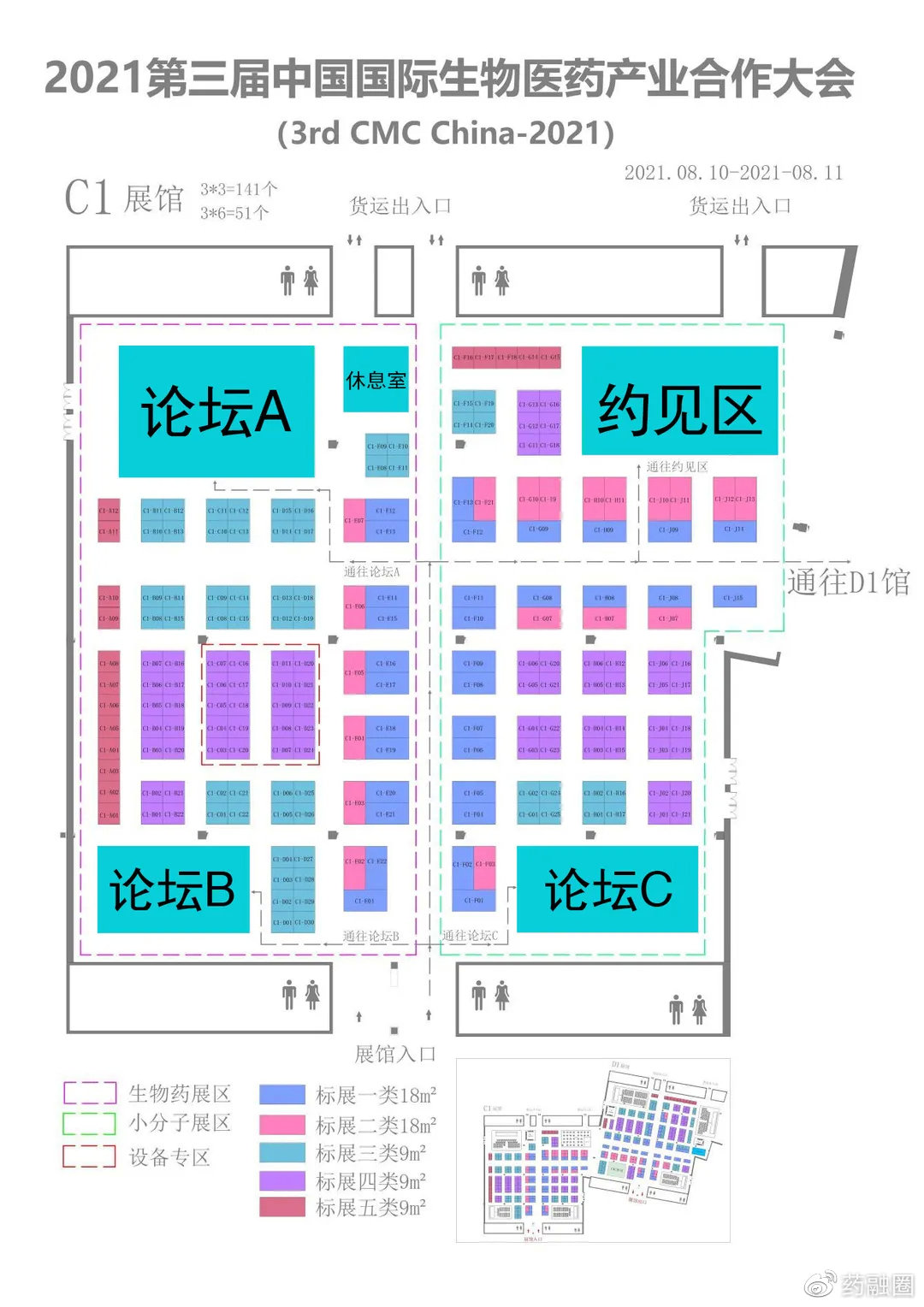
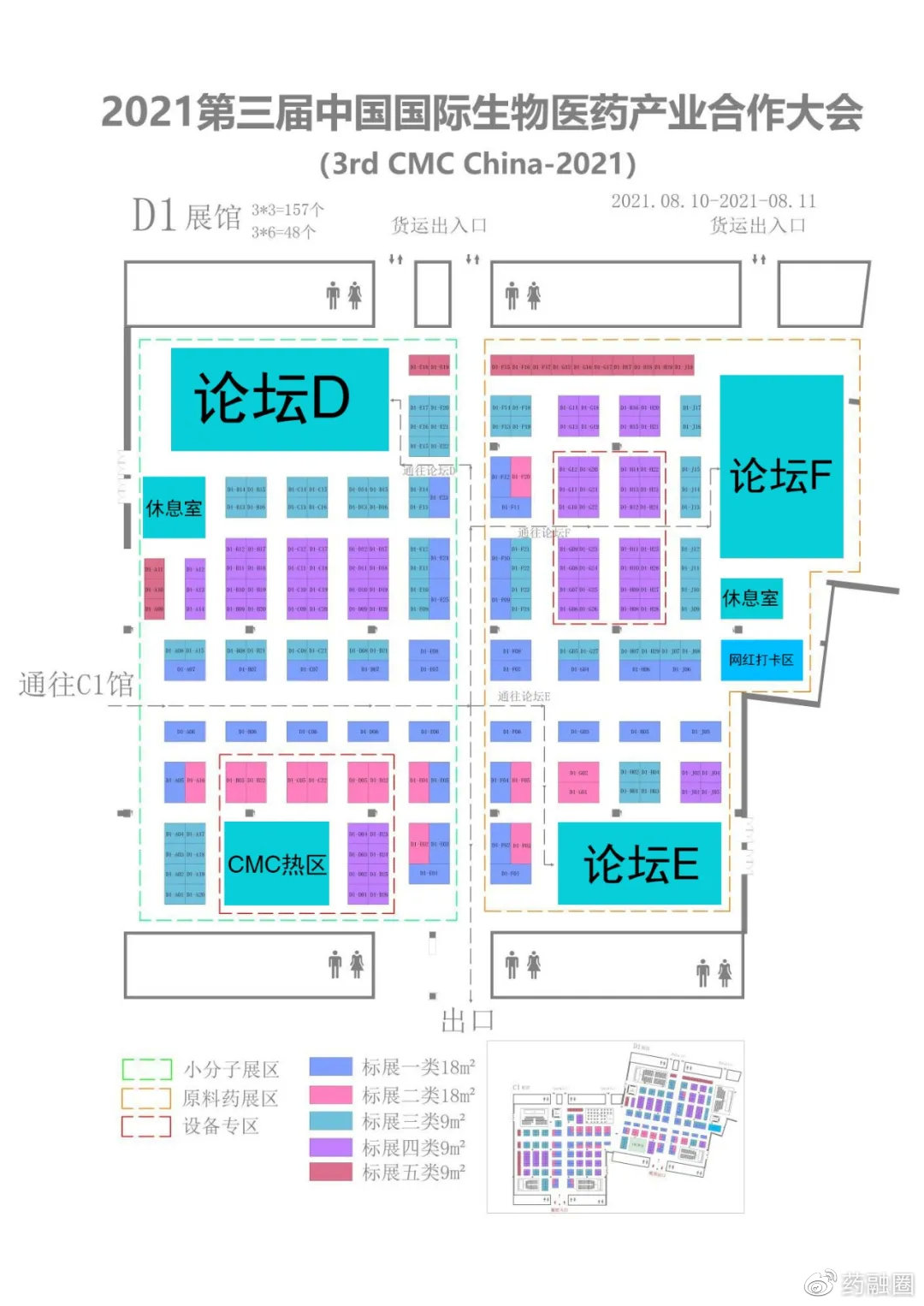
As the market changes and policies are implemented, traditional chemical drug companies are upgrading and transforming, new Biotech companies are mushrooming, and CXO companies related to preclinical, clinical and CMC are also booming.China has gradually entered the situation of generic drugs and innovative drugs, chemical drugs and biological drugs.Transnational cooperation License in/out, overseas mergers and acquisitions are also common.
Against this backdrop, we and hundreds of pharmaceutical enterprises jointly hold The 3rd China International Biological & Chemical Pharmaceutical Industry Conference(The 3rd CMC-China 2021) in Suzhou, the largest city of pharmaceutical industry. Six theme forums are set up at the conference, covering the whole field of macro-molecules & small molecules, generic and innovative drugs, upper & down streams chain of active pharmaceutical ingredients (API) and pharmaceutical preparations.
At the 3rd CMC-China conference, you will get pharmaceutical industrial policy interpretation and cutting-edge technology sharing, covering antibody drugs, vaccines and mRNA technology, innovative drugs and generic drugs project approval, research and development, clinical and production, green chemical actual combat technology, etc. At the conference, you can find more business opportunities in the area of active pharmaceutical ingredients (API), pharmaceutical intermediates and pharmaceutical excipients, packaging materials, instruments, equipment, consumables, as well as pharmaceutical & clinical CRO, CMO and MAH related projects. At the conference, you will meet a group of industry friends and partners!
Post time: Sep-28-2021

