OEM Factory for Valsartan - Irinotecan Hydrochloride CAS 100286-90-6 Purity ≥99.0% (HPLC) API USP Standard High Purity – Ruifu
OEM Factory for Valsartan - Irinotecan Hydrochloride CAS 100286-90-6 Purity ≥99.0% (HPLC) API USP Standard High Purity – Ruifu Detail:
Manufacturer Supply Irinotecan and Related Intermediates:
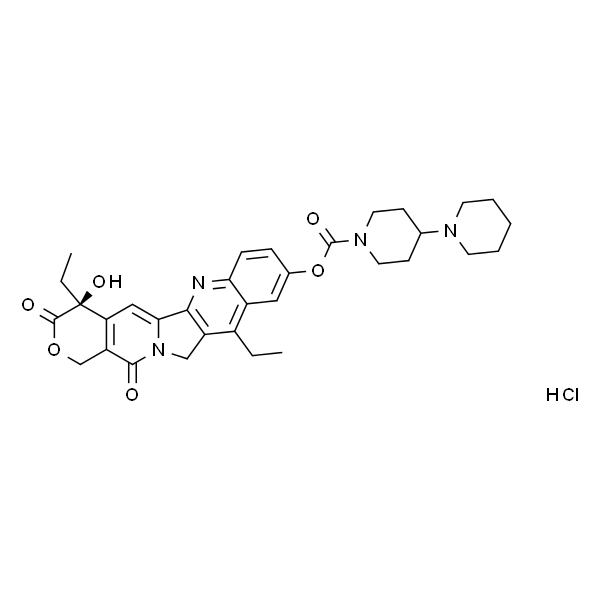
Irinotecan Hydrochloride CAS: 100286-90-6
Irinotecan Free Base CAS: 97682-44-5
Irinotecan Hydrochloride Trihydrate CAS: 136572-09-3
7-Ethyl-10-Hydroxycamptothecin CAS: 86639-52-3
1-Chlorocarbonyl-4-Piperidinopiperidine Hydrochloride CAS: 143254-82-4
| Chemical Name | Irinotecan Hydrochloride |
| Synonyms | Camptothecin II; CPT-II |
| CAS Number | 100286-90-6 |
| CAT Number | RF-API50 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C33H39ClN4O6 |
| Molecular Weight | 623.15 |
| Melting Point | 250-256℃ (dec.) |
| Storage Conditions | Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Powder |
| Solubility | Soluble in water and methanol, slightly soluble in chloroform, ethanol |
| Identification IR | The infrared absorption spectrum of the test sample should consistent with that of the reference standard |
| Identification HPLC | The retention time of major peak of the sample solution should correspond to that of the reference standard. |
| Identification | Meets the requirements of the tests for Chloride Positive reaction |
| Moisture (K.F) | 7.0%~9.0% w/w |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤20ppm |
| Irinotecan Hydrochloride Enantiomer | ≤0.10% (HPLC) |
| 7-Desethyl Irinotecan | ≤0.15% |
| Irinotecan Related Compound A | ≤0.15% |
| 11-Ethyl Irinotecan | ≤0.15% |
| Camptothecin | ≤0.15% |
| Irinotecan Related Compound B | ≤0.15% |
| 7-Ethylcanptothecin | ≤0.15% |
| 7,11-Diethyl-10-Hydroxy Camptothecin | ≤0.15% |
| Any Unspecified Impurity | ≤0.10% |
| Total Impurities | ≤0.50% |
| Residual Solvents (GC) | |
| Methanol | ≤2000ppm |
| Acetone | ≤2000ppm |
| Dichloromethane | ≤500ppm |
| Peteoleum Ether: | ≤100ppm |
| Ethyl Acetate | ≤2000ppm |
| Benzene | ≤2ppm |
| Bacterial Endotoxins | ≤0.29 EU/mg Irinotecan |
| Microbial Limits | Total Aerobic Microbial Count: <1000 cfu/g |
| Molds and Yeasts | <100 cfu/g |
| Purity / Analysis Method | ≥99.0% (HPLC, on the anhydrous basis) |
| Test Standard | United States Pharmacopeia (USP) Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


lrinotecan hydrochloride (CAS 100286-90-6), a semi-synthetic, water soluble derivative of the potent anticancer agent camptothecin, was launched in Japan for the treatment of lung, ovarian, and cervical cancers. lrinotecan exerts its antitumor activity via inhibition of topoisomerase I, a cellular enzyme that is involved in maintaining the topographic structure of DNA during the process of translation, transcription, and mitosis. lrinotecan undergoes de-esterification in vivo to yield an active metabolite, SN-38, which is 1000-fold more potent than the parent. Combination therapy of irinotecan with another widely used anticancer agent, cisplatin, has been reported to be superior to either agent alone. lrinotecan is in clinical trials for gastrointestinal, breast, skin, colorectal, pancreatic cancers, mesothelioma and non-Hodgkin’s lymphoma.
Product detail pictures:
Related Product Guide:
"Quality initial, Honesty as base, Sincere support and mutual profit" is our idea, so as to build repeatedly and pursue the excellence for OEM Factory for Valsartan - Irinotecan Hydrochloride CAS 100286-90-6 Purity ≥99.0% (HPLC) API USP Standard High Purity – Ruifu , The product will supply to all over the world, such as: Sierra Leone, Guinea, Cannes, Our tenet is "integrity first, quality best". We have confidence in providing you with excellent service and ideal products. We sincerely hope we can establish win-win business cooperation with you in the future!
-
Best Price on Gemcitabine Hydrochloride - Omep...
-
OEM manufacturer Rivaroxaban Intermediates - 4...
-
Excellent quality 2-(5-Bromo-2-methylbenzyl)-5-...
-
Factory selling Methyl D-(-)-Mandelate - (R)-1...
-
Hot sale Factory 4-(4-Aminophenoxy)-N-Methylpic...
-
Competitive Price for 2-(4-Methoxyphenyl)ethyla...
We always believe that the details decides the company's product quality, in this respect, the company conform our requirements and the goods are meet our expectations.
