Olaparib AZD-2281 CAS 763113-22-0 Purity ≥99.0% API Factory
High Purity, Commercial Production
Olaparib and Related Intermediates:
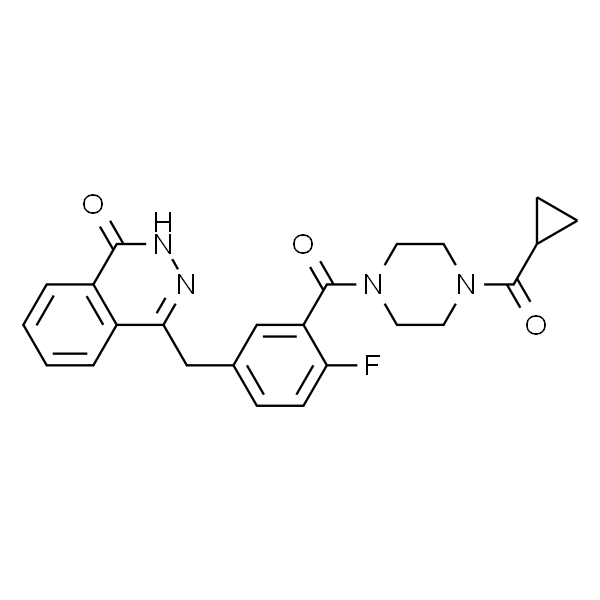
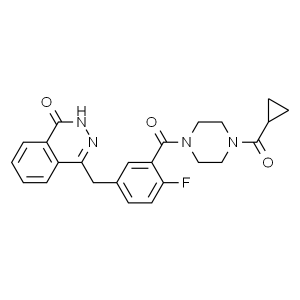
Olaparib CAS 763113-22-0
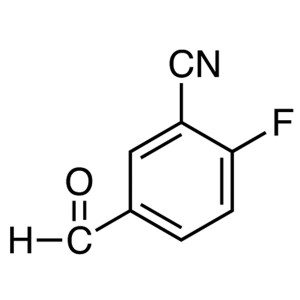
2-Fluoro-5-Formylbenzonitrile CAS 218301-22-5
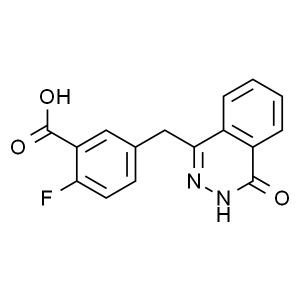
2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid CAS 763114-26-7
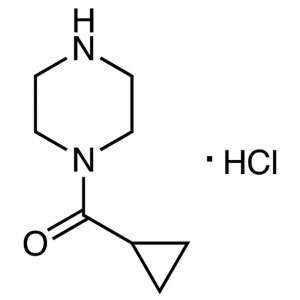
1-(Cyclopropylcarbonyl)piperazine Hydrochloride CAS 1021298-67-8
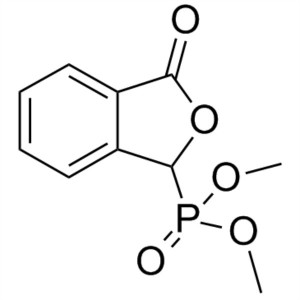
3-Oxo-1,3-Dihydroisobenzofuran-1-Ylphosphonic Acid CAS 61260-15-9
| Chemical Name | Olaparib |
| Synonyms | AZD-2281; KU0059436; Lynparza; 4-(3-(4-(cyclopropanecarbonyl)piperazine-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; 1-(Cyclopropylcarbonyl)-4-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)Methyl]-2-fluorobenzoyl]piperazin |
| CAS Number | 763113-22-0 |
| CAT Number | RF-API103 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C24H23FN4O3 |
| Molecular Weight | 434.46 |
| Solubility | Soluble in DMSO |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Identification by 1H NMR | Comply with the structure |
| LC-MS | Comply with the structure |
| Purity / Analysis Method | ≥99.0% (by LC-MS) |
| Moisture (K.F) | ≤0.50% |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Heavy Metals (as Pb) | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | API; PARP Inhibitor |
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


Olaparib (CAS: 763113-22-0), a highly potent and selective PARP-inhibitor. In December 19, 2014, the FDA approved novel anti-cancer drug Olaparib (Lynparza) for monotherapy to the patients of advanced ovarian cancer who has undergone at least 3 rounds of chemotherapy or patients of suspected BRCA mutations. At the same time, FDA approved the quantitation and classification of diagnostic kits for the detection of mutations in BRCA1 and BRCA2, BRACAnalysis CDx. Olaparib is the first PARP inhibitor drugs which has been approved by FDA. In February 2, 2015, the European Union Food and Drug Administration (EMA) also approved Olaparib to enter into market in the 28 countries of European Union including Iceland, Liechtenstein and Norway. But the indications of EMA and FDA approved are slightly different; the former is for the BRCA gene mutation cases, and also for the maintenance therapy for patients of advanced epithelial ovarian cancer who has previously received platinum-containing chemotherapy drugs and exhibit response and subject to recurrence.