Oxcarbazepine CAS 28721-07-5 Assay >99.0% API Anticonvulsant
Leading Supplier of Oxcarbazepine Intermediates
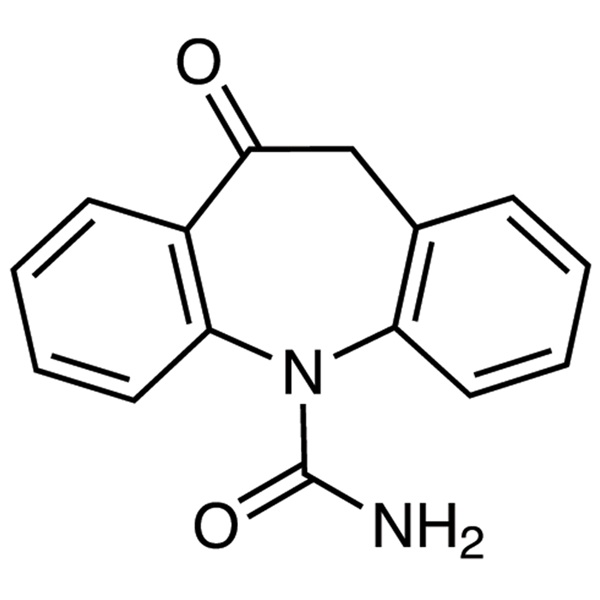
Oxcarbazepine CAS 28721-07-5
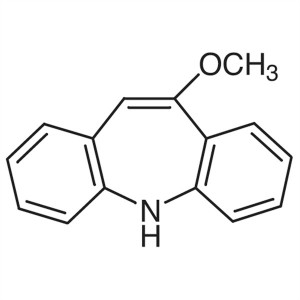
10-Methoxyiminostilbene CAS 4698-11-7
Please Contact: alvin@ruifuchem.com
| Chemical Name | Oxcarbazepine |
| Synonyms | 10,11-Dihydro-10-oxo-5H-Dibenzo[b,f]azepine-5-Carboxamide; Aurene; GP 47680; Oxecarb; Oxetol; Trileptal |
| CAS Number | 28721-07-5 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C15H12N2O2 |
| Molecular Weight | 252.27 |
| Melting Point | 222.0~228.0℃(dec.)(lit.) |
| Boiling Point | 457.2±55.0℃ |
| Density | 1.329±0.06 g/cm3 |
| Water Solubility | Insoluble in Water |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications | Results |
| Appearance | White or Pale Yellow Crystalline Powder | Conforms |
| Identification | IR & HPLC | Conforms |
| Assay / Analysis Method | >99.0% | 99.67% |
| Melting Point | 222.0~228.0℃ | 223.0~ 224.0℃ |
| Loss on Drying | <0.30% | 0.21% |
| Residue on Ignition | <0.10% | 0.08% |
| Related Substances | ||
| Carbamazepine | <0.20% | 0.14% |
| Other Single Impurity | <0.20% | <0.20% |
| Total Unknown Impurities | <0.50% | <0.50% |
| Total Impurities | <1.00% | 0.33% |
| Test Standard | Enterprise Standard | Conforms |
| Application | Anticonvulsant |
Package: Bottle, Aluminum foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture
How to Purchase? Please contact: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Russia, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes R22 - Harmful if swallowed
R36 - Irritating to the eyes
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
R11 - Highly Flammable
Safety Description S16 - Keep away from sources of ignition.
S36/37 - Wear suitable protective clothing and gloves.
UN IDs UN 1648 3 / PGII
WGK Germany 3
RTECS HN8445000
Oxcarbazepine (CAS: 28721-07-5) is an anticonvulsant drug primarily used in the treatment of epilepsy. There is some evidence for oxcarbazepine as a mood-stabilizing agent and thus, it can be used as add-on therapy for bipolar disorder in patients that have failed or are unable to tolerate approved treatments. Oxcarbazepine is used alone or in combination with other medications to control certain types of seizures. Oxcarbazepine is in a class of medications called anticonvulsants. It works by decreasing abnormal electrical activity in the brain. It is indicated as monotherapy or adjunctive therapy for partial seizures in adults with epilepsy, as monotherapy for the treatment of partial seizures in children 4 years of age or older, and as adjunct therapy in children 2 to 4 years of age. Oxcarbazepine is chemically and pharmacologically closely related to carbamazepine, but it has much less capacity to induce drug-metabolizing enzymes. This property decreases the problems associated with drug interactions when oxcarbazepine is used in combination with other drugs. The clinical uses and adverse effect profile of oxcarbazepine appear to be similar to those of carbamazepine. Oxcarbazepine was approved for use as an anticonvulsant in Denmark in 1990, Spain in 1993, Portugal in 1997, and eventually for all other EU countries in 1999. It was approved in the US in 2000. Oxcarbazepine is a second-generation AED supplied under the proprietary brand name of Trileptal® (Novartis, Basel) in the UK and USA.
Patients with hypersensitivity reactions to carbamazepine can be expected to show cross-sensitivity (e.g., rash) or related problems to oxcarbazepine. The improved toxicity profile for oxcarbazepine when compared to CBZ may result from absence of the epoxide or CBZ-iminoquinone metabolites. The most common side effects are headache, dizziness, nystagmus, blurred vision, somnolence, nausea, ataxia, and fatigue. The incidence of adverse effects has been related to elevated serum MHD concentrations. Adverse effects on cognitive status, hyponatremia, and serious dermatological reactions have been reported, as has hyponatremia.
Oxcarbazepine
C15H12N2O2 252.27
5H-Dibenz[b,f]azepine-5-carboxamide, 10,11-dihydro-10-oxo-;
10,11-Dihydro-10-oxo-5H-dibenz[b,f]azepine-5-carboxamide [28721-07-5].
DEFINITION
Oxcarbazepine contains NLT 98.0% and NMT 102.0% of C15H12N2O2, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption <197K> [Note-If the spectrum obtained shows differences, dissolve the substance to be examined in chloroform, and evaporate to dryness. Compare the spectrum of the residue to that of a similarly prepared USP Oxcarbazepine RS. ]
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Buffer: 6.8 g/L of monobasic potassium phosphate in water. For each L prepared add 2 mL of triethlyamine and mix. Adjust with phosphoric acid to a pH of 6.0 ± 0.1.
Mobile phase: Methanol, acetonitrile, and Buffer (11:8:31)
Standard solution: 0.1 mg/mL of USP Oxcarbazepine RS in Mobile phase
Sample solution: 0.1 mg/mL of Oxcarbazepine in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 50
Flow rate: 1.5 mL/min
Injection size: 10 µL
System suitability
Sample: Standard solution
Suitability requirements:
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C15H12N2O2 in the portion of Oxcarbazepine taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Oxcarbazepine RS in the Standard solution (mg/mL)
CU = concentration of Oxcarbazepine in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition 281: NMT 0.1%
• Heavy Metals, Method II <231>: 10 ppm
SPECIFIC TESTS
• Water Determination, Method Ia <921>: NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed containers. Store at controlled room temperature.
• USP Reference Standards <11>
USP Oxcarbazepine RS