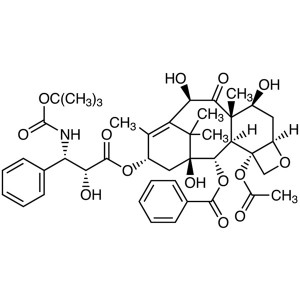
Paclitaxel (Taxol) CAS 33069-62-4 Assay (HPLC) 97.0~102.0% Factory High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Paclitaxel (Taxol) (CAS: 33069-62-4) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Paclitaxel (Taxol), Please contact: alvin@ruifuchem.com
| Chemical Name | Paclitaxel |
| Synonyms | Taxol; Abraxane; Genaxol; Genetaxyl; OncoGel; Pacliex; (-)-Paclitaxel |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 33069-62-4 |
| Molecular Formula | C47H51NO14 |
| Molecular Weight | 853.92 g/mol |
| Melting Point | 211℃(dec.) |
| Density | 0.200 |
| Water Solubility | Insoluble in Water |
| Solubility | Easily Soluble in Chloroform, Acetone and Other Organic Solvents. |
| Stability | Stable. Incompatible with Strong Oxidizing Agents. Combustible. |
| COA & MSDS | Available |
| Sample | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Crystalline Powder, Odorless | Conforms |
| Solubility | Practically insoluble in water, soluble in methanol and freely soluble in methylene chloride |
Conforms |
| Identification (IR) | Infrared Absorption: In accordance with the reference standard spectrum of paclitaxel |
Conforms |
| Identification (HPLC) | The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay |
Conforms |
| Specific Rotation | -49.0° to -55.0° | -52.3° |
| Related Substances | ||
| Baccatin III | ≤0.20% | 0.03% |
| 10-Deacetylpaclitaxel | ≤0.50% | 0.08% |
| 10-Debenzoylbaccatin III | ≤0.10% | 0.05% |
| Photodegradant | ≤0.10% | Not Detected |
| 2-Debenzoylpaclitaxel-2-pentenoate | ≤0.70% | Not Detected |
| Sum of X1, X2, X3 | ≤0.40% Oxetan ring opened, acetyl and bezoyl midrated X1 10-Acetoacetylpaclitaxel X2 10-Deacetyl-7-Epipaclitaxel (Paclitaxel Related Compound B) X3 |
Not Detected |
| 10,13-Bissidechainpaclitaxel | ≤0.50% | Not Detected |
| 7-Acetylpaclitaxel | ≤0.60% | Not Detected |
| 7-Tes-Paclitaxel | ≤0.30% | Not Detected |
| 13-Tes-Baccatin III | ≤0.10% | Not Detected |
| 7-Epipaclitaxel | ≤0.40% | 0.032% |
| Any Unspecified Impurity | ≤0.10% | Not Detected |
| Total Impurities | ≤2.00% | 0.032% |
| Residual Organic Solvents | ||
| Acetone | ≤5000ppm | 360ppm |
| Heptane | ≤5000ppm | 180ppm |
| Methanol | ≤300ppm | Not Detected |
| Ethyl Acetate | ≤300ppm | Not Detected |
| Ethanol | ≤5000ppm | Not Detected |
| Dichloromethane | ≤600ppm | Not Detected |
| Water by Karl Fischer | ≤4.00% | 0.90% |
| Residue on Ignition | ≤0.20% | 0.09% |
| Heavy Metals (Pb) | ≤20ppm | Conforms |
| Microbial Limit Tests | ||
| The Total Acrobic Bactria | ≤20cfu/g | Conforms |
| The Total Yersts and Molds | ≤20cfu/g | Conforms |
| Escherichia Coli | Not Detected | Conforms |
| Staphylococcus Aureus | Not Detected | Conforms |
| Saimonella | Not Detected | Conforms |
| Pseudomonas Acruginosa | Not Detected | Conforms |
| Bacterial Endotoxins | ≤0.4EU/mg | Conforms |
| Assay (HPLC) | 97.0~102.0% (calculated on the anhydrous, solvent-free basis) | 99.9% |
| Conclusion | The product has been tested and complies with the USP43 Paclitaxel | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Paclitaxel
C47H51NO14 853.91
Benzenepropanoic acid, -(benzoylamino)--hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, [2aR-[2a,4,4a,6,9(R*,S*),11,12,12a,12b]]-.
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]-benz[1,2-b]oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine [33069-62-4].
Paclitaxel contains not less than 97.0 percent and not more than 102.0 percent of C47H51NO14, calculated on the anhydrous, solvent-free basis.
[Caution-Paclitaxel is cytotoxic. Great care should be taken to prevent inhaling particles of Paclitaxel and exposing the skin to it.]
Packaging and storage-Preserve in tight, light-resistant containers, and store at controlled room temperature.
Labeling-The labeling indicates the type of process used to produce the material and the Related compounds test with which the material complies.
USP Reference standards <11>-
USP Endotoxin RS
USP Paclitaxel RS Click to View Structure
USP Paclitaxel Related Compound A RS
Cephalomannine.
USP Paclitaxel Related Compound B RS
10-Deacetyl-7-Epipaclitaxel.
USP Paclitaxel Impurity Mixture RS
Mixture of Paclitaxel and the following related compounds: propyl analog, cephalomannine, sec-butyl analog, n-butyl analog, benzyl analog, baccatin VI, pentyl analog, and 7-epipaclitaxel.
Identification-
A: Infrared Absorption <197K>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Specific rotation 781S: between 49.0 and 55.0 at 20, calculated on the anhydrous, solvent-free basis.
Test solution: 10 mg per mL, in methanol.
Microbial enumeration tests <61> and Tests for specified microorganisms <62>-The total aerobic microbial count does not exceed 100 cfu per g. It meets the requirements of the tests for the absence of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella species, and Escherichia coli.
Bacterial endotoxins 85-It contains not more than 0.4 USP Endotoxin Unit per mg of paclitaxel.
Water, Method Ic <921>: not more than 4.0%.
Residue on ignition <281>: not more than 0.2%.
Heavy metals, Method II <231>: 0.002%.
Related Compounds-
test 1 (for material labeled as isolated from natural sources)-If the material complies with this test, the labeling indicates that it meets USP Related compounds Test 1.
Diluent-Prepare as directed in the Assay.
Solution A-Prepare filtered and degassed acetonitrile.
Solution B-Prepare filtered and degassed water.
Mobile phase-Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography 621).
System suitability solution-Dissolve accurately weighed quantities of USP Paclitaxel Related Compound A RS and USP Paclitaxel Related Compound B RS in methanol to obtain a solution having known concentrations of about 10 µg of each per mL. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, dilute with Diluent to volume, and mix.
Standard solution-Dissolve, with the aid of sonication, an accurately weighed quantity of USP Paclitaxel RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 5 µg per mL.
Test solution-Use the Assay preparation.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 227-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L43. The flow rate is about 2.6 mL per minute. The column temperature is maintained at 30. The chromatograph is programmed as follows.
Time (minutes) Solution A (%) Solution B (%) Elution
0–35 35 65 isocratic
35–60 35®80 65®20 linear gradient
60–70 80®35 20®65 linear gradient
70–80 35 65 isocraticChromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.78 for paclitaxel related compound A and 0.86 for paclitaxel related compound B (relative to the retention time for paclitaxel obtained from the Test solution); and the resolution, R, between paclitaxel related compound A and paclitaxel related compound B is not less than 1.0. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure-Inject a volume (about 15 µL) of the Test solution into the chromatograph, record the chromatogram, and measure the areas for the major peaks. Calculate the percentage of each impurity in the portion of Paclitaxel taken by the formula:
100(Fri / rU)
in which F is the relative response factor for each impurity peak (see Table 1 for values); ri is the peak area for each individual impurity; and rU is the peak area for paclitaxel.
Table 1
Relative Retention Time Relative Response Factor (F) Name Limit (%)
0.24 1.29 Baccatin III 0.2
0.53 1.00 10-Deacetylpaclitaxel 0.5
0.57 1.00 7-Xylosylpaclitaxel 0.2
0.78 1.26 Cephalomannine (Paclitaxel related compound A) a11
0.78 1.26 2'',3''-Dihydrocephalomannine a21
0.86 1.00 10-Deacetyl-7-epipaclitaxel (Paclitaxel related compound B) 0.5
1.10 1.00 Benzyl analog3 b12
1.10 1.00 3'',4''-Dehydropaclitaxel C b22
1.40 1.00 7-Epicephalomannine 0.3
1.85 1.00 7-Epipaclitaxel 0.5
1 Resolution may be incomplete for these peaks, depending upon the relative amounts present; the sum of a1 and a2 is not more than 0.5%.
2 Resolution may be incomplete for these peaks, depending upon the relative amounts present; the sum of b1 and b2 is not more than 0.5%.
3 The following chemical name is assigned to the related compound, benzyl analog:Baccatin III 13-ester with (2R,3S)-2-hydroxy-3-phenyl-3-(2-phenylacetylamino)propanoic acid.
In addition to not exceeding the limits for paclitaxel related impurities in Table 1, not more than 0.1% of any other single impurity is found; and not more than 2.0% of total impurities is found.
test 2 (for material labeled as produced by a semisynthetic process)— If the material complies with this test, the labeling indicates that it meets USP Related compounds Test 2.
Diluent-Use acetonitrile.
Solution A-Use a filtered and degassed mixture of water and acetonitrile (3:2).
Solution B-Use filtered and degassed acetonitrile.
Mobile phase-Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography 621).
System suitability solution-Dissolve accurately weighed quantities of USP Paclitaxel RS and USP Paclitaxel Related Compound B RS in Diluent, shaking and sonicating if necessary, to obtain a solution having known concentrations of about 0.96 mg and 0.008 mg per mL, respectively.
Test solution-Transfer about 10 mg of Paclitaxel, accurately weighed, to a 10-mL volumetric flask, dissolve in and dilute with Diluent to volume, shaking and sonicating if necessary, and mix.
Chromatographic system (see Chromatography 621)— The liquid chromatograph is equipped with a 227-nm detector and a 4.6-mm × 15-cm column that contains 3-µm packing L1. The flow rate is about 1.2 mL per minute. The column temperature is maintained at 35. The chromatograph is programmed as follows.
Time (minutes) Solution A (%) Solution B (%) Elution
0-20 100 0 isocratic
20-60 100®10 0®90 linear gradient
60-62 10®100 90®0 linear gradient
62-70 100 0 isocraticChromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.94 for paclitaxel related compound B and 1.0 for paclitaxel; the resolution, R, between paclitaxel related compound B and paclitaxel is not less than 1.2; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure-Separately inject equal volumes (about 15 µL) of the Diluent and the Test solution into the chromatograph, record the chromatograms, and measure the areas for all the peaks. Disregard any peaks due to the Diluent. Calculate the percentage of each impurity in the portion of Paclitaxel taken by the formula:
100(Fri / rs)
in which F is the relative response factor for each impurity (see Table 2 for values); ri is the peak area for each impurity obtained from the Test solution; and rs is the sum of the areas of all the peaks obtained from the Test solution.
Table 2
Relative Retention Time Relative Response Factor (F) Name Limit (%)
0.11 1.24 10-Deacetylbaccatin III 0.1
0.20 1.29 Baccatin III 0.2
0.42 1.39 Photodegradant2 0.1
0.47 1.00 10-Deacetylpaclitaxel 0.5
0.80 1.00 2-Debenzoylpaclitaxel-2-pentenoate 0.7
0.921 1.00 Oxetane ring opened, acetyl and benzoyl migrated2 x1
0.921 1.00 10-Acetoacetylpaclitaxel x2
0.941 1.00 10-Deacetyl-7-epipaclitaxel (Paclitaxel related compound B) x3
1.37 1.00 7-Epipaclitaxel 0.4
1.45 1.00 10,13-Bissidechainpaclitaxel2 0.5
1.54 1.00 7-Acetylpaclitaxel 0.6
1.80 1.75 13-Tes-baccatin III 0.1
2.14 1.00 7-Tes-paclitaxel 0.3
1 Resolution may be incomplete for these peaks, depending upon the relative amounts present; the sum of x1, x2, and x3 is not more than 0.4%.
2 The following chemical names are assigned to the related compounds Photodegradant; Oxetane ring opened, acetyl and benzoyl migrated; and 10,13-Bissidechainpaclitaxel:
Photodegradant
(1R,2R,4S,5S,7R,10S,11R,12S,13S,15S,16S)-2,10-diacetyloxy-5,13-dihydroxy-4,16,17,17-tetramethyl-8-oxa-3-oxo-12-phenylcarbonyloxypentacyclo[11.3.1.01,11.04,11.07,10]heptadec-15-yl
(2R,3S)-2-hydroxy-3-phenyl-3-(phenylcarbonylamino)propanoate
Oxetane ring opened, acetyl and benzoyl migrated
(1S,2S,3R,4S,5S,7S,8S,10R,13S)-5,10-diacetyloxy-1,2,4,7-tetrahydroxy-8,12,15,15-tetramethyl-9-oxo-4-(phenylcarbonyloxymethyl)tricyclo[9.3.1.03,8]pentadec-11-en-13-yl
(2R,3S)-2-hydroxy-3-phenyl-3-(phenylcarbonylamino)propanoate
10,13-Bissidechainpaclitaxel
Baccatin III 13-ester with (2R,3S)-2-hydroxy-3-phenyl-3-(phenylcarbonylamino)propanoic acid, 10-ester with (2S,3S)-2-hydroxy-3-phenyl-3-(phenylcarbonylamino)propanoic acid
In addition to not exceeding the limits for paclitaxel related impurities in Table 2, not more than 0.1% of any other single impurity is found; and not more than 2.0% of total impurities is found.
test 3 (for material labeled as produced by a plant cell fermentation process)— If the material complies with this test, the labeling indicates that it meets USP Related compounds Test 3.
Solution A-Prepare a filtered and degassed mixture of water and acetonitrile (3:2).
Solution B-Prepare filtered and degassed acetonitrile.
Mobile phase-Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography 621).
System suitability solution-Dissolve USP Paclitaxel Impurity Mixture RS in acetonitrile, sonicating if necessary, to obtain a solution having a known concentration of about 1 mg per mL.
Standard solution-Dissolve an accurately weighed quantity of USP Paclitaxel RS in acetonitrile, sonicating if necessary, to obtain a solution having a known concentration of about 1 mg per mL.
Test solution-Transfer about 10 mg of Paclitaxel, accurately weighed, to a 10-mL volumetric flask. Dissolve in and dilute with acetonitrile to volume, sonicating if necessary, and mix.
Chromatographic system (see Chromatography 621)— The liquid chromatograph is equipped with a 227-nm detector and a 4.6-mm × 15-cm column that contains 3-µm packing L1. The flow rate is about 1.2 mL per minute. The chromatograph is programmed as follows.
Time (minutes) Solution A (%) Solution B (%) Elution
0-28 100 0 isocratic
28-33 100®98 0®2 linear gradient
33–58 98®10 2®90 linear gradient
58-60 10 90 isocratic
60-63 10®100 90®0 linear gradient
63-70 100 0 isocraticChromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between paclitaxel and benzyl analog is not less than 1.8. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%. [note—For the purpose of peak identification, the approximate relative retention times are given in Table 3. The relative retention times are measured versus Paclitaxel. ]
Table 3
Name Relative Retention Time Limit (%)
Propyl analog1 0.54 0.2
Cephalomannine (Paclitaxel related compound A) 0.76 0.5
sec-Butyl analog2 0.81 0.2
n-Butyl analog3 0.89 0.1
Benzyl analog 1.10 0.4
Baccatin VI 1.23 0.2
Pentyl analog4 1.31 0.2
7-Epipaclitaxel 1.51 0.4
1 The following chemical name is assigned to the related compound Propyl analog:Baccatin III 13-ester with (2R,3S)-3-butanoylamino-2-hydroxy-3-phenylpropanoic acid.
2 The following chemical name is assigned to the related compound sec-Butyl analog:Baccatin III 13-ester with (2R,3S)-2-hydroxy-3-(2-methylbutanoylamino)-3-phenylpropanoic acid.
3 The following chemical name is assigned to the related compound n-Butyl analog:Baccatin III 13-ester with (2S,3S)-2-hydroxy-3-(pentanoylamino)-3-phenylpropanoic acid.
4 The following chemical name is assigned to the related compound Pentyl analog:Baccatin III 13-ester with (2R,3S)-3-(hexanoylamino)-2-hydroxy-3-phenylpropanoic acid.
Procedure-Inject a volume (about 12 µL) of the Test solution into the chromatograph, record the chromatogram, and measure the areas for all the peaks. Calculate the percentage of each impurity in the portion of Paclitaxel taken by the formula:
100(ri / rU)
in which ri is the response of each individual impurity; and rU is the sum of the areas of all the peaks obtained from the Test solution. In addition to not exceeding the limits for paclitaxel related impurities in Table 3, not more than 0.1% of any other single impurity is found; and not more than 2.0% of total impurities is found.
Assay-
Diluent-Prepare a mixture of methanol and acetic acid (200:1).
Mobile phase-Prepare a filtered and degassed mixture of water and acetonitrile (11:9). Make adjustments if necessary (see System Suitability under Chromatography 621).
Standard preparation-Dissolve, using sonication if necessary, an accurately weighed quantity of USP Paclitaxel RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 1 mg per mL.
Assay preparation-Transfer about 10 mg of Paclitaxel, accurately weighed, to a 10-mL volumetric flask. Dissolve in Diluent, using sonication if necessary, dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 227-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L43. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is between 0.7 and 1.3; and the relative standard deviation for replicate injections is not more than 1.5%.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of C47H51NO14 in the portion of Paclitaxel taken by the formula:
10C(rU / rS)
in which C is the concentration, in mg per mL, of USP Paclitaxel RS in the Standard preparation; and rU and rS are the peak responses for paclitaxel obtained from the Assay preparation and the Standard preparation, respectively.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xn - Harmful
Risk Codes R37/38 - Irritating to respiratory system and skin.
R41 - Risk of serious damage to eyes
R42/43 - May cause sensitization by inhalation and skin contact.
R62 - Possible risk of impaired fertility
R68 - Possible risk of irreversible effects
R40 - Limited evidence of a carcinogenic effect
R48 - Danger of serious damage to health by prolonged exposure
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
R68/20/21/22 -
Safety Description S22 - Do not breathe dust.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
UN IDs 1544
WGK Germany 3
RTECS DA8340700
FLUKA BRAND F CODES 10-21
HS Code 2932999021
Hazard Class 6.1(b)
Packing Group III
Toxicity LD50 intraperitoneal in mouse: 128mg/kg
Paclitaxel (Taxol) (CAS: 33069-62-4), a natural product isolated from the bark of the Pacific yew, is effective in treating refractory metastatic ovarian cancer. Unlike any other antineoplastic agents, paclitaxel appears to have several possible mechanisms of action, including an antimicrotubule action through the promotion of tubulin polymerization and stabilization of microtubules, thereby, halting mitosis and promoting cell death. The supply of paclitaxel is limited by its low natural abundance and currently it is being manufactured by a semi-synthetic route from deacetylbaccatin Ⅲ that is isolated from the needles of the yew tree. Recent completion of two total syntheses of taxol conquered the structural complexity of the title compound and may be useful in obtaining certain closely related analogs, some of which have been found to have antitumor activity. Paclitaxel has potential uses in the treatment of metastatic breast cancer, lung cancer, head and neck cancer, and malignant melanoma.
The toxic ingredients in branches and leaves of Taxus chinensis were separated in 1856 and named “taxine,” which was identified as a kind of white alkaloid’s component. Currently, among all the antitumor drugs, the sale of paclitaxel becomes the first in the world as a well-recognized anticancer drug with potent broad-spectrum activity. In October of 1995, China became the second country with formal production of paclitaxel and its injection in the world. The achievement was gained under the unremitting efforts of researchers in the Institute of Materia Medica, Chinese Academy of Medical Sciences.
Paclitaxel (CAS: 33069-62-4) is an antineoplastic that used to treat patients with lung, ovarian, breast cancer, head and neck cancer, and advanc ed forms of Kaposi's sarcoma. Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It is also used in the study of structure and function of microtubles into tubulin.
Paclitaxel has a good effect on refractory ovarian cancer and breast cancer with drug resistance such as ovarian cancer and platinum, and a good prospect for the treatment of prostate cancer, head and neck cancer, esophageal cancer, germ cell tumor, endometrial cancer, lymphoma, bladder cancer, upper digestive tract cancer, small cell and non-small cell lung cancer.
Paclitaxel (Taxol) (CAS: 33069-62-4) is a highly complex, organic compound isolated from the bark of the Pacific yew tree. It binds to tubulin dimers and microtubulin filaments, promoting the assembly of filaments and preventing their depolymerization. This increase in the stability of microfilaments results in disruption of mitosis and cytotoxicity and disrupts other normal microtubular functions, such as axonal transport in nerve fibers. The major mechanism of resistance that has been identified for paclitaxel is transport out of tumor cells, which leads to decreased intracellular drug accumulation. This form of resistance is mediated by the multidrug transporter P-glycoprotein.
May be sensitive to prolonged exposure to moisture.