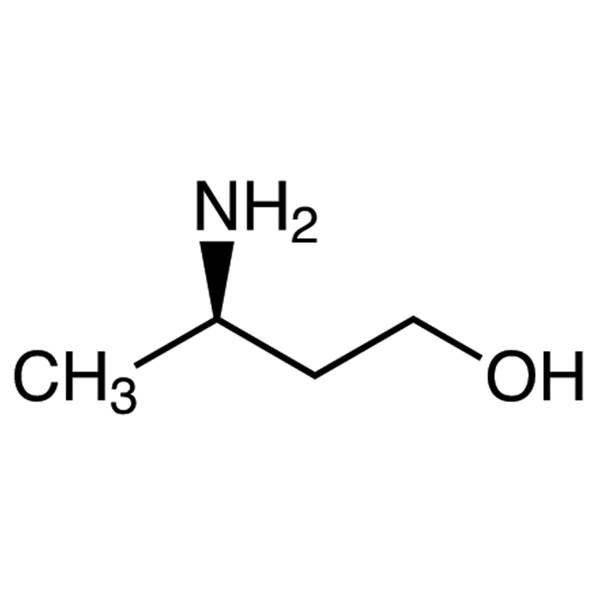
(R)-3-Amino-1-Butanol CAS 61477-40-5 Purity >99.0% (GC) Dolutegravir Intermediate Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (R)-3-Amino-1-Butanol (CAS: 61477-40-5) with high quality, commercial production. It's an intermediate of Dolutegravir (CAS: 1051375-16-6). We can provide Certificate of Analysis (COA), worldwide delivery, small and bulk quantities available, strong after-sale service. Please contact: alvin@ruifuchem.com
| Chemical Name | (R)-3-Amino-1-Butanol |
| Synonyms | (R)-3-Aminobutan-1-ol; (3R)-3-Amino-1-Butanol |
| CAS Number | 61477-40-5 |
| CAT Number | RF-CC317 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C4H11NO |
| Molecular Weight | 89.14 |
| Specific Gravity (20/20) | 0.95 |
| Refractive Index | 1.45 |
| Specific Rotation [α]20/D | -12.0° ~ -10.0° (C=1, EtOH) |
| Brand | Ruifu Chemical |
|
Appearance |
Colorless to Light Yellow Liquid |
Light Yellow Liquid |
|
Identification by IR |
Conforms to Spectrum of the Standard |
Conforms |
|
Identification by GC |
Conforms to Retention Time of the Standard |
Conforms |
|
Purity by GC (A%) |
>99.0% |
99.8% |
|
Related Substances |
Any Single Impurity: <0.5% |
0.09% |
|
Total Impurities: <1.0% |
0.28% |
|
|
S-Isomer (by HPLC A%) |
<0.10% |
0.02% |
|
Water Content by KF |
<0.50% |
0.10% |
|
Assay by GC (w/w%) |
>99.0% |
99.3% |
Package: Fluorinated Bottle, 25kg/Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


(R)-3-Amino-1-Butanol (CAS: 61477-40-5) can be used as an intermediate in the preparation of compounds having HIV integrase inhibitory activity. (R)-3-Amino-1-Butanol is an intermediate of Dolutegravir (CAS: 1051375-16-6). Dolutegravir (Tivicay) was a new kind of anti-ADIS drug that jointly developed by the British pharmaceutical giant GlaxoSmithKline (GSK) with the Japanese Shionogi Pharmaceutical Company (Shionogi). In August 2013, the US FDA approved dolutegravir (also referred to as S/GSK1349572) for the treatment of HIV-1 infection in adults and children ages 12 years and older in combination with other antiretroviral drugs.
-
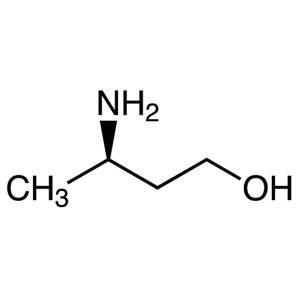
(R)-3-Amino-1-Butanol CAS 61477-40-5 Purity >99...
-
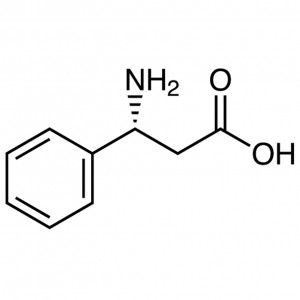
(R)-3-Amino-3-Phenylpropionic Acid CAS 13921-90...
-
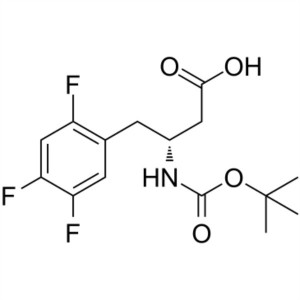
Boc-(R)-3-Amino-4-(2,4,5-Trifluoro-Phenyl)-Buty...
-
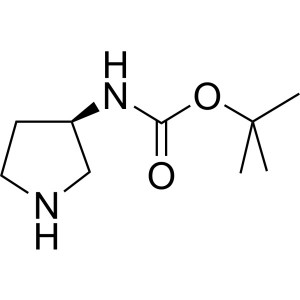
(R)-3-(Boc-Amino)pyrrolidine CAS 122536-77-0 Pu...
-
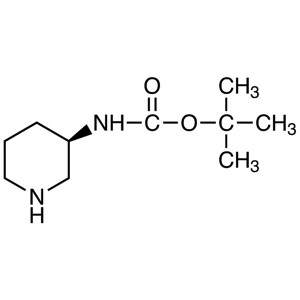
(R)-3-(Boc-Amino)piperidine CAS 309956-78-3 Lin...
-
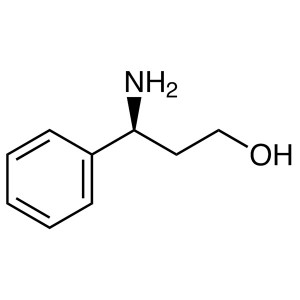
(S)-3-Amino-3-Phenylpropan-1-ol CAS 82769-76-4 ...
-
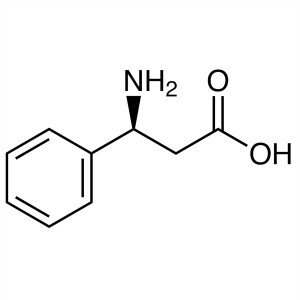
(S)-3-Amino-3-Phenylpropanoic Acid CAS 40856-44...
-
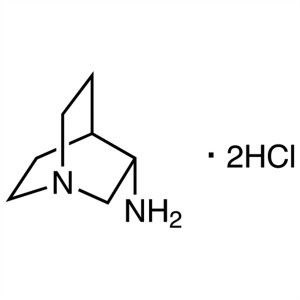
(S)-3-Aminoquinuclidine Dihydrochloride CAS 119...
-
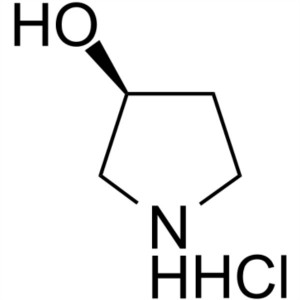
(S)-3-Hydroxypyrrolidine Hydrochloride CAS 1225...
-
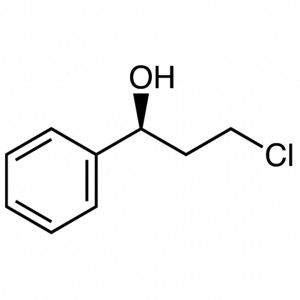
(S)-(-)-3-Chloro-1-Phenyl-1-Propanol CAS 100306...
-
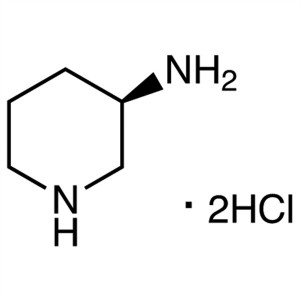
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 3...
-
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 3...
-
(R)-(-)-3-Carbamoymethyl-5-Methylhexanoic Acid ...
-
(R)-(-)-3-Chloro-1,2-Propanediol CAS 57090-45-6...
-
(R)-(-)-3-Piperidinecarboxylic Acid CAS 25137-0...
-
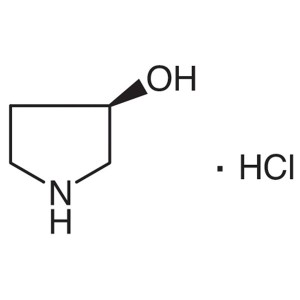
(R)-(-)-3-Pyrrolidinol Hydrochloride CAS 104706...