Remdesivir GS-5734 CAS 1809249-37-3 COVID-19 API High Quality
Manufacturer Commercial Supply Remdesivir and Related Intermediates with High Quality
Remdesivir CAS 1809249-37-3
2-Ethyl-1-butanol CAS 97-95-0
Trimethylsilyl Cyanide CAS 7677-24-9
4-Nitrophenol CAS 100-02-7
2-Ethylbutyl ((S)-(perfluorophenoxy)(phenoxy)phosphoryl)-L-alaninate CAS 1911578-98-7
N-[(S)-(4-nitrophenoxy)phenoxyphosphinyl]-L-Alanine 2-ethylbutyl ester CAS 1354823-36-1
(S)-2-Ethylbutyl 2-Aminopropanoate Hydrochloride CAS 946511-97-3
Remdesivir Metabolite (GS-441524) CAS 1191237-69-0
Remdesivir N-2 Intermediate CAS 1191237-80-5
2,3,5-Tri-O-benzyl-D-ribonolactone CAS 55094-52-5
7-Bromopyrrolo[2,1-f][1,2,4]triazin-4-amine CAS 937046-98-5
Pyrrolo[1,2-F][1,2,4]Triazin-4-Amine CAS 159326-68-8
4-Amino-7-iodopyrrolo[2,1-f][1,2,4]triazine CAS 1770840-43-1
| Chemical Name | Remdesivir |
| Synonyms | GS-5734; 2-ethylbutyl ((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)-L-alaninate |
| CAS Number | 1809249-37-3 |
| CAT Number | RF-API96 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C27H35N6O8P |
| Molecular Weight | 602.58 |
| Density | 1.47±0.1 g/cm3 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Solid Powder |
| Identification A | IR: The infrared absorption spectrum of the product should be consistent with that of the reference substance |
| Identification B | HPLC Retention time similar to reference substance |
| Specific Rotation | -19.0° ~ -22.0° |
| Solubility | Solunle in Methanol, Sparingly Soluble in Ethanol, Slightly Soluble in Acetonitrile, Practically Insoluble in Water |
| Related Substances | |
| Any Individual Impurity | ≤0.10% |
| Total Impurities | ≤1.0% |
| RD-3.1 | ≤0.10% |
| RD-C | ≤0.10% |
| RD-D | ≤0.10% |
| RD-E | ≤0.10% |
| RP-Isomer | ≤0.10% |
| Nitrophenol | ≤0.10% |
| Residual Solvents | |
| Methanol Dichloride | ≤6000ppm |
| Acetone | ≤5000ppm |
| Isopropyl Alcohol | ≤5000ppm |
| Acetonitrile | ≤410ppm |
| Dichloromethane | ≤600ppm |
| Methyl tert Butyl Ether | ≤5000ppm |
| Ethyl Acetate | ≤5000ppm |
| Tetrahydrofuran | ≤720ppm |
| n-Heptane | ≤5000ppm |
| Assay | 98.0%~102.0% (Calculated on the dried basis) |
| Total Aerobic Counts | |
| Aerobic Bacteria | ≤100cfu/g |
| Yeast and Mould | ≤10cfu/g |
| E. Coil | Negative |
| Bacterial Endotoxin | ≤1.0EU |
| Heavy Metals | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | API, COVID-19 |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Remdesivir (CAS 1809249-37-3), sold under the brand name Veklury, is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. During the COVID-19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID-19 in around 50 countries. Updated guidelines from the World Health Organization in November 2020 include a conditional recommendation against the use of Remdesivir for the treatment of COVID-19. Remdesivir was originally developed to treat hepatitis C, and was subsequently investigated for Ebola virus disease and Marburg virus infections before being studied as a post-infection treatment for COVID-19. The most common side effect in healthy volunteers is raised blood levels of liver enzymes (a sign of liver problems). The most common side effect in people with COVID-19 is nausea. Side effects may include liver inflammation and an infusion-related reaction with nausea, low blood pressure, and sweating. Remdesivir is a prodrug that is intended to allow intracellular delivery of GS-441524 monophosphate and subsequent biotransformation into GS-441524 triphosphate, a ribonucleotide analogue inhibitor of viral RNA polymerase.
-
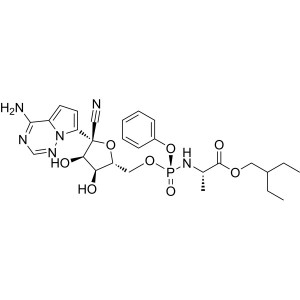
Remdesivir GS-5734 CAS 1809249-37-3 COVID-19 AP...
-
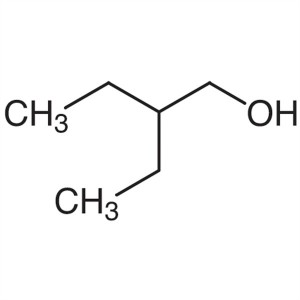
2-Ethyl-1-butanol CAS 97-95-0 Remdesivir Interm...
-
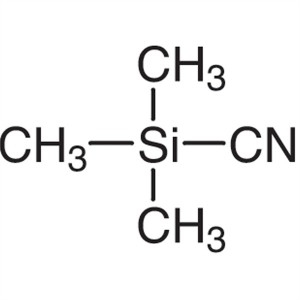
Trimethylsilyl Cyanide TMSCN CAS 7677-24-9 Assa...
-
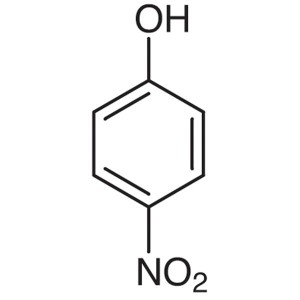
4-Nitrophenol CAS 100-02-7 High Quality Factory
-
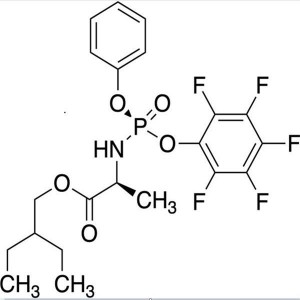
Remdesivir Intermediate CAS 1911578-98-7 2-Ethy...
-
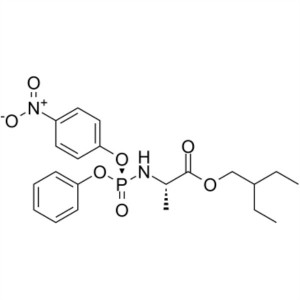
Remdesivir Intermediate CAS 1354823-36-1 COVID-19
-
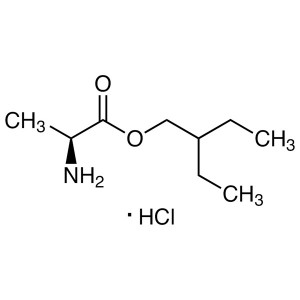
Remdesivir Intermediate CAS 946511-97-3 COVID-1...
-
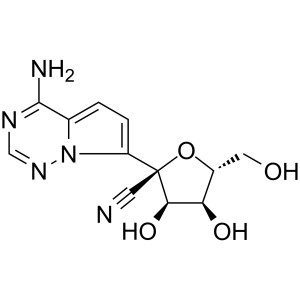
Remdesivir Metabolite (GS-441524) CAS 1191237-6...
-
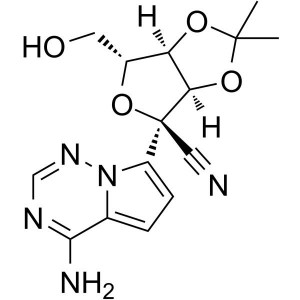
Remdesivir N-2 Intermediate CAS 1191237-80-5 CO...
-
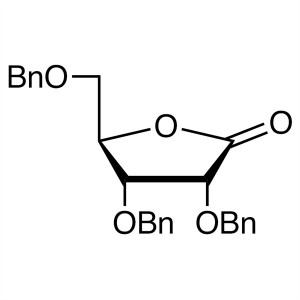
2,3,5-Tri-O-benzyl-D-ribonolactone CAS 55094-52...
-
![7-Bromopyrrolo[2,1-f][1,2,4]triazin-4-amine CAS 937046-98-5 Remdesivir Intermediate COVID-19](https://www.ruifuchem.com/uploads/7-Bromopyrrolo21-f124triazin-4-amine-CAS-937046-98-51-300x300.jpg)
7-Bromopyrrolo[2,1-f][1,2,4]triazin-4-amine CAS...
-
![Pyrrolo[1,2-F][1,2,4]Triazin-4-Amine CAS 159326-68-8 Remdesivir Intermediate COVID-19](https://www.ruifuchem.com/uploads/CAS-159326-68-8-300x300.png)
Pyrrolo[1,2-F][1,2,4]Triazin-4-Amine CAS 159326...
-
![Remdesivir Intermediate CAS 1770840-43-1 4-Amino-7-iodopyrrolo[2,1-f][1,2,4]triazine COVID-19](https://www.ruifuchem.com/uploads/Remdesivir-Intermediate-CAS-1770840-43-1-4-Amino-7-iodopyrrolo21-f124triazine-COVID-19-300x300.jpg)
Remdesivir Intermediate CAS 1770840-43-1 4-Amin...

