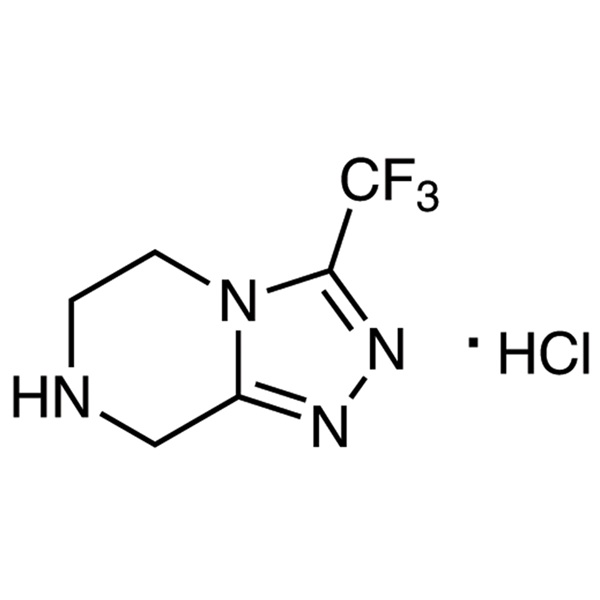
Sitagliptin Triazole Hydrochloride CAS 762240-92-6 Purity >99.0% (HPLC) Factory High Quality
Supply Sitagliptin Phosphate Monohydrate Related Intermediates:
Sitagliptin API CAS 486460-32-6
Sitagliptin Phosphate Monohydrate API CAS 654671-77-9
2,4,5-Trifluorophenylacetic Acid CAS 209995-38-0
Boc-(R)-3-Amino-4-(2,4,5-Trifluoro-Phenyl)-Butyric Acid CAS 486460-00-8
Sitagliptin Triazole Hydrochloride CAS 762240-92-6
Sitagliptin Phosphate Monohydrate Intermediate CAS 486460-21-3
| Chemical Name | 3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine Hydrochloride |
| Synonyms | Sitagliptin Triazole Hydrochloride; Sitagliptin Intermediate A |
| CAS Number | 762240-92-6 |
| CAT Number | RF-PI1193 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H7F3N4·HCl |
| Molecular Weight | 228.60 |
| Melting Point | 236.0~246.0℃ |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification by HPLC | The retention time of sample is concordant with reference standard |
| Identification by IR | Complies |
| Loss on Drying | <0.50% |
| Related Substances | |
| Any Single Impurity | <0.50% |
| Total Impurities | <1.00% |
| Heavy Metals | <20ppm |
| Purity / Analysis Method | >99.0% (HPLC) |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Sitagliptin Phosphate Monohydrate (CAS: 654671-77-9) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine Hydrochloride (CAS: 762240-92-6) is an intermediate of Sitagliptin Phosphate Monohydrate (CAS: 654671-77-9), which is an orally-bioavailable selective DPP4 inhibitor that was discovered through the optimization of a class of β-aminoacid-derived DPP4 inhibitors. It lowers DPP4 activity in a sustained manner following once daily administration, preserves the circulating levels of intact GIP and GLP1 following meals in both acute and chronic studies and reduces blood glucose levels without significant increases in hypoglycaemia. Sitagliptin Phosphate (STG) is used to treat DM type 2 because it improves glycemic control by increasing the levels of active incretin hormones, GLP-1 (peptide-1) and GIP (glucose-dependent insulinotropic peptide). STG was approved by the FDA in 2006.
-
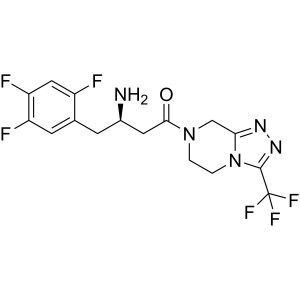
Sitagliptin CAS 486460-32-6 Purity >99.0% (HPLC...
-
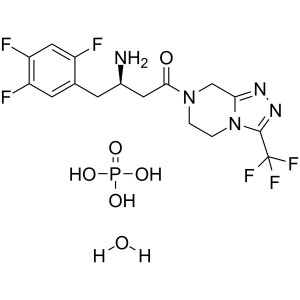
Sitagliptin Phosphate Monohydrate CAS 654671-77...
-
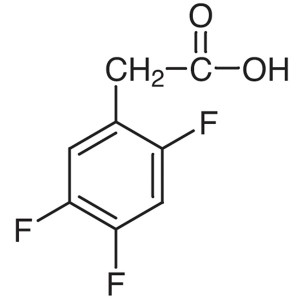
2,4,5-Trifluorophenylacetic Acid CAS 209995-38-...
-
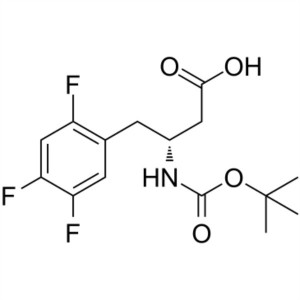
Boc-(R)-3-Amino-4-(2,4,5-Trifluoro-Phenyl)-Buty...
-
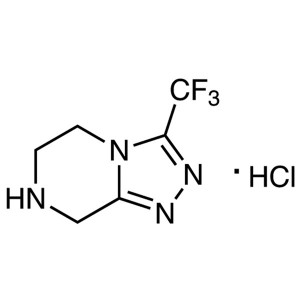
Sitagliptin Triazole Hydrochloride CAS 762240-9...
-
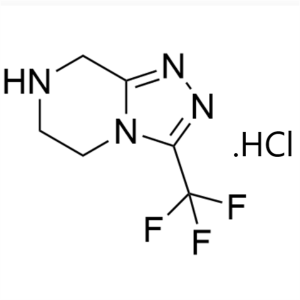
Sitagliptin Phosphate Monohydrate Intermediate ...