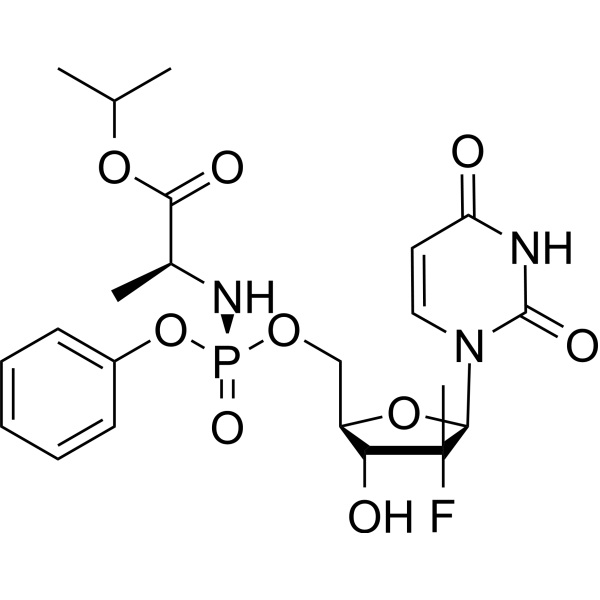
Sofosbuvir CAS 1190307-88-0 Purity ≥99.0% (HPLC)
| Name | Sofosbuvir |
| Synonyms | GS-7977; PSI-7977 |
| CAS Number | 1190307-88-0 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C22H29FN3O9P |
| Molecular Weight | 529.45 |
| Melting Point | 120.0~125.0℃ |
| Density | 1.41 |
| Solubility | Soluble in DMF, DMSO, Ethanol |
| Shipping Condition | Under Ambient Temperature |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Shelf Life | 36 Months if Stored Properly |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification | HPLC; RT |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| E.E | ≥99.0% |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.20% |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Heavy Metals | ≤20ppm |
| Residual Solvents | Meet the requirements of specifications |
| Methanol | ≤1500ppm |
| TBME | ≤2500ppm |
| Dichloromethane | ≤720ppm |
| Tetrahydrofuran | ≤720ppm |
| Toluene | ≤60ppm |
| Test Standard | Enterprise Standard |
| Usage | Sofosbuvir (CAS: 1190307-88-0) in the treatment of Hepatitis C Virus (HCV) |
Package: Bottle, Aluminum foil bag, 25kg/Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture

Sofosbuvir (CAS: 1190307-88-0) (formally labeled as PSI-797, GS-7977) is a phosphoramidate prodrug and nucleotide polymerase inhibitor for the treatment of chronic hepatitis C virus (HCV) infection. It is recommended to be used in combination with other drugs (such as velpatasvir) for the first-line treatment for HCV genotypes 1, 2, 3, 4, 5, and 6. It takes effect through acting as a nucleotide analog inhibitor, being capable of specially inhibiting the HCV NS5B (non-structural protein 5B) RNA-dependent RNA polymerase.
Sofosbuvir is the world's first oral treatment of hepatitis C drugs and is 2013 in the United States One of the most important new drugs approved can eliminate the need for the traditional injection drug interferon (IFN) when used in the treatment of chronic hepatitis C of a specific genotype, successfully developed by Gilead Science, the world's largest manufacturer of anti-HIV drugs, with the English name Sofosbuvir and Sovaldi, its global sales peak may exceed $10 billion.