Teneligliptin Hydrobromide Intermediate CAS 401564-36-1 Purity >99.5% (HPLC) Factory
Supply Teneligliptin Hydrobromide Related Intermediates With High Purity
Teneligliptin Hydrobromide CAS 906093-29-6
1-(3-Methyl-1-Phenyl-5-Pyrazolyl)piperazine CAS 401566-79-8
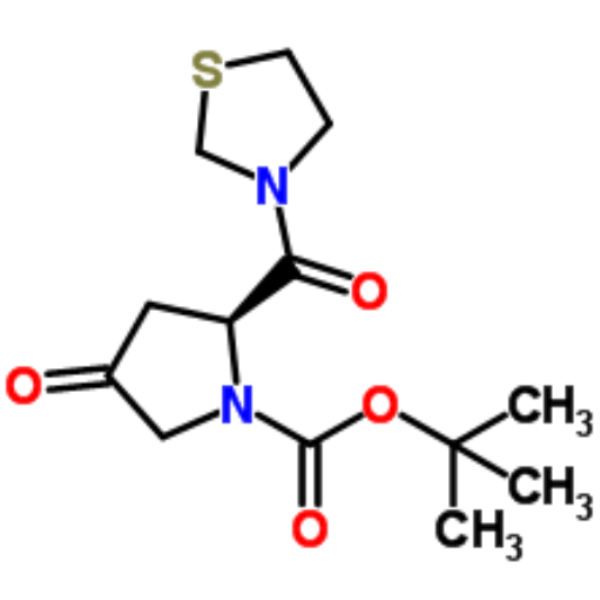
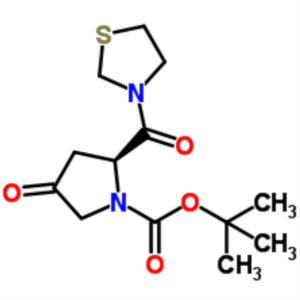
Teneligliptin Hydrobromide Intermediate CAS 401564-36-1
| Chemical Name | (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester |
| Synonyms | (S)-tert-Butyl 4-oxo-2-(thiazolidine-3-carbonyl)pyrrolidine-1-carboxylate; 3-((S)-1-tert-butoxycarbonyl-4-oxo-2-pyrrolidinylcarbonyl)-1,3-thiazolidine; (2S)-4-oxo-2-(1,3-thiazolidin-3-ylcarbonyl)pyrrolidine-1-carboxylate; Teneligptin Intermediate B |
| CAS Number | 401564-36-1 |
| CAT Number | RF-1818 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C13H20N2O4S |
| Molecular Weight | 300.37 |
| Density | 1.305 |
| Brand | Ruifu Chemical |
| Item | Inspection Standard | Results |
| Appearance | White to Cream Coloured Solid | Conform |
| Identification | IR; HPLC RT | Conform |
| Loss on Drying | <1.00% | 0.20% |
| Related Substances | Any Single Impurity <0.50% | 0.24% |
| Total Impurity <0.50% | 0.39% | |
| Assay | 99.5%~102.0% (On Dried Basis) | 99.8% |
| Enantiomeric Purity | >99.5% | 99.9% |
| Sulphated Ash | <0.20% | 0.02% |
| Test Standard | Enterprise Standard | |
| Usage | Intermediates of Teneligliptin Hydrobromide (CAS: 906093-29-6) | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester (CAS: 401564-36-1) is an intermediate used to prepare dipeptidyl peptidase IV (DPP-IV) inhibitor, intermediates of Teneligliptin Hydrobromide (CAS: 906093-29-6). Teneligliptin is a DPP-4 inhibitor which was approved in Japan in 2012 for the treatment of type II diabetes. It was discovered and developed by Mitsubishi Tanabe Pharma under the trade name Tenelia®. Similar to other marketed DPP-4 inhibitors, Teneligliptin was well tolerated in all studies and QD dosing produced a long-lasting inhibitory action against DPP-4 and an increase in active GLP-1 levels, with very low rates of renal excretion.