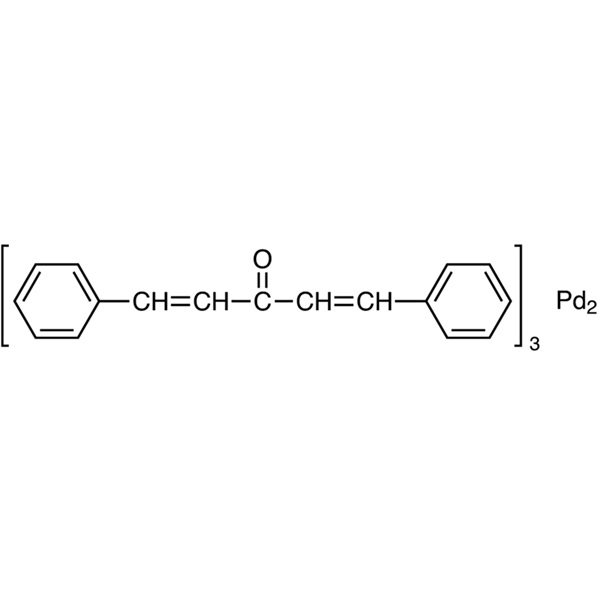
Tris(dibenzylideneacetone)dipalladium(0) CAS 51364-51-3 Assay >97.0% Pd 20.9~21.9%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Tris(dibenzylideneacetone)dipalladium(0) (CAS: 51364-51-3) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | Tris(dibenzylideneacetone)dipalladium(0) |
| Synonyms | [Pd2(dba)3] x dba; Pd2dba3; Pd2(dba)3; Tris(dba)dipalladium(0); Bis[tris(dibenzylideneacetone)palladium(0)] |
| CAS Number | 51364-51-3 |
| CAT Number | RF-PI2210 |
| Stock Status | In Stock |
| Molecular Formula | C51H42O3Pd2 |
| Molecular Weight | 915.73 |
| Melting Point | 152.0~155.0℃ |
| Sensitive | Light Sensitive, Air Sensitive, Moisture Sensitive |
| Water Solubility | Insoluble in Water |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Black Purple Powder |
| Assay | >97.0% |
| Palladium Content (Pd) | 20.9~21.9% (ICP) |
| Carbon by Elemental Analysis | 64.6~69.2% |
| Total Metallic Impurities | ≤1600ppm |
| A Single Impurity Content | ≤100ppm |
| Infrared Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Shelf Life | 24 Months |
Package: Bottle, Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Tris(dibenzylideneacetone)dipalladium(0), synonyms Pd2(dba)3, (CAS: 51364-51-3) is a cycloaddition catalyst, is the most widely used Pd0 precursor complex in synthesis and catalysis, in particular as a catalyst for various coupling reactions. It is used as catalyst for a wide variety of Pd catalyzed reactions including Suzuki coupling, Heck coupling, Negishi coupling, Carroll reaarangement, Trost asymmetric allylic alkylation, Buchwald-Hartwig amination of acryl halides, fluorination of allylic chlorides, arylation of ketones, carbonylation of 1,1-dichloro-1-alkenes, ß-arylation of carboxylic esters, and conversion of aryl and vinyl triflates to aryl and vinyl halides. It is also involved in the synthesis of azepane. Tris(dibenzylideneacetone)dipalladium(0) is used in the preparation of semiconducting polymers processed from nonchlorinated solvents into high performance thin film transistors. Also used in the synthesis of polymer bulk-heterojunction solar sells as a semiconductor. Its worked related to trace metals basis, Asymmetric synthesis, Catalysis or catalyst, Coupling reactions, Cross couplings, Heck Reaction, Ligands,Organic synthesis,Suzuki coupling, Polymer science, Materials Chemistry, Column chromatography and Organic Compounds, and Arylations, Aminations, Deprotonations, Hydroxylations, Fluorinations. Catalyst precursor for conversion of aryl chlorides, triflates, and nonaflates to nitroaromatics. Catalyst for the synthesis of epoxides. Catalytic asymmetric allylic and homoallylic diamination of terminal olefins. Site-selective benzylic sp3 palladium-catalyzed direct arylation. Palladium-catalyzed one-pot synthesis of tricyclic indolines. Active catalyst for the Suzuki-Miyaura coupling of 2-pyridyl nucleophiles. Catalyst in combination with BINAP for the asymmetric Heck Arylation of olefins. Precursor for palladium-catalyzed carbon-nitrigen bond formation. Catalyst for α-arylation of ketones. Cross-coupling of aryl halides with aryl boronic acids.
-
Tris(dibenzylideneacetone)dipalladium(0) CAS 51...
-
Bis(dibenzylideneacetone)palladium(0) CAS 32005...
-
Bis(tri-tert-butylphosphine)palladium(0) CAS 53...
-
Palladium CAS 7440-05-3 Pd ≥9.75%
-

Palladium on Activated Charcoal 10% Pd CAS 6474...
-
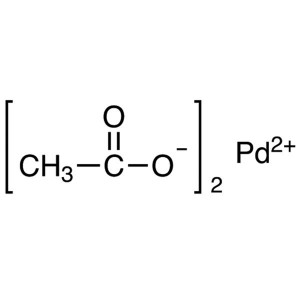
Palladium(II) Acetate CAS 3375-31-3 Purity >99....
-
Palladium(II) Bromide CAS 13444-94-5 Purity >98...
-
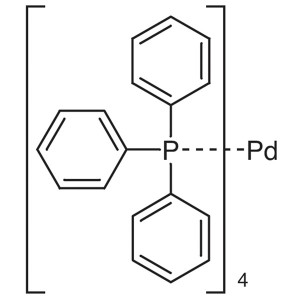
Tetrakis(triphenylphosphine)palladium(0) CAS 14...
-
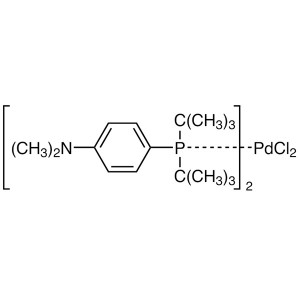
Pd(AmPhos)2Cl2 CAS 887919-35-9 Assay >98.0%
-
Sodium Tetrachloropalladate(II) CAS 13820-53-6 ...